Rhopalomyia solidaginis ( Loew 1862 )
|
publication ID |
https://doi.org/ 10.5281/zenodo.188745 |
|
DOI |
https://doi.org/10.5281/zenodo.6217077 |
|
persistent identifier |
https://treatment.plazi.org/id/074287C9-FFD2-E30D-FF01-FF755EC63DAB |
|
treatment provided by |
Plazi |
|
scientific name |
Rhopalomyia solidaginis ( Loew 1862 ) |
| status |
|
Rhopalomyia solidaginis ( Loew 1862) View in CoL
Cecidomyia solidaginis Loew 1862: 194 View in CoL ; Felt 1915: 246 ( Rhopalomyia View in CoL ). Rhopalomyia albipennis Felt 1908: 364 View in CoL . New synonym.
Rhopalomyia carolina Felt 1908: 363 View in CoL . New synonym.
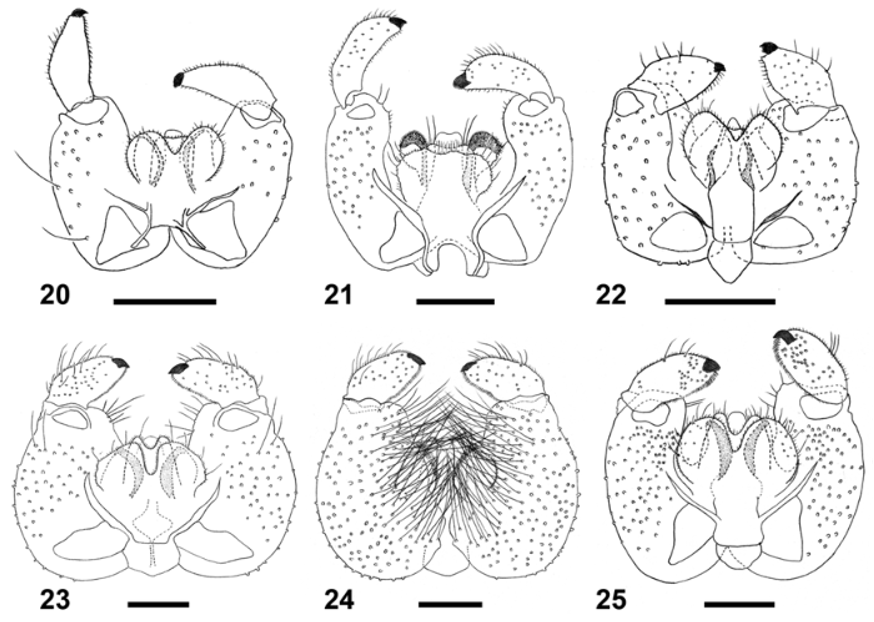
Adult: Body densely covered by dark scales. Antenna with 17 or 19–20 flagellomeres in male (spring and summer generations, respectively), 17 or 20–23 flagellomeres in female (spring and summer generations, respectively); necks of male flagellomeres III–VII 0.63–1.00 or 0.50–0.80 times as long as nodes (spring and summer generations respectively); female flagellomeres without necks. Palpus 2-segmented; second segment at least twice as long as first, rounded or sometimes tapered apically, setose and setulose, with several elongate, dark brown scales. Wing length 3.7 or 4.0– 4.2 mm in male (spring and summer generations, respectively), 3.1–3.3 or 2.8–4.1 in female (spring and summer generations, respectively). Legs densely covered by dark brown scales. Male terminalia ( Figs. 23–24 View FIGURES 20 – 25 ): gonocoxite bulbous and robust, very wide at mid length, strongly setose, with very long, mesally directed setae on ventral side ( Fig. 24 View FIGURES 20 – 25 ), with strongly setose mediobasal lobe; gonocoxal apodeme undivided, narrows anteriorly; gonostylus short and wide, narrows only slightly toward apex, setose and setulose throughout, with brush-like tooth; aedeagus rounded apically; hypoproct with apical notch, setose and setulose; cerci very wide, separated by deep notch, strongly setose and setulose. Female abdomen (Fig. 38): tergite 7 trapezoid, weakly sclerotized along anterior and lateral margins, with two anterior trichoid sensilla, several rows of setae posteriorly, and groups of setae mesolaterally; tergite 8 Y-shaped, proximal arms narrow distally and widen proximally, less than third the length of shaft, each with anterior trichoid sensillum; shaft with weakly sclerotized patches; ovipositor 4.0–4.1 or 3.6–4.6 times as long as tergite 7 (spring and summer generations, respectively).
Pupa ( Figs. 54–55 View FIGURES 48 – 55 ): Light orange. Antennal bases short and blunt; basal part wide V-shaped in frontal view. Frons sometimes with tiny projection at mid length, without pronounced lateral projections; basal edge rounded. Prothoracic spiracle divided apically into 2–3 lobes.
Type material: Cecidomyia solidaginis Loew. Neotype designated here: male, USA, West Dryden, NY, 28/V/1987, M.V. McEvoy, reared from rosette gall on S. altissima . The neotype is designated in order to clarify the taxonomic status of R. solidaginis (Loew) , whose type series is lost. The neotype is so labeled and is deposited together with 9 associated permanent microscopic slides of 7 females and 7 males of the same series in the USNM. This species was based on 1 male and 1 female from USA, District of Columbia, collected by B. Osten Sacken in August (unspecified date), ex. S. altissima . These syntypes are neither in the MCZC nor in the USNM and we consider them lost.
Rhopalomyia albipennis Felt : Syntypes: 1 male, USA, Albany, NY, 10–11/IX/1907, E.P. Felt, Felt # a1655, deposited in Felt Collection; 2 males (pinned), USA, Albany, NY, 14/IX/1907, E.P. Felt, Felt # a1655, deposited in Felt Collection.
Rhopalomyia carolina Felt : Holotype: 1 female, USA, Asheville, NC, 4/X/1906, E.P. Felt, Felt # a1635, deposited in Felt Collection.
Other material examined (all from Solidago altissima unless otherwise noted): R. albipennis : 1 female, USA, Bath, NY, 5/IX/1907, E.P. Felt, Felt # a1655, deposited in Felt Collection; 1 female, USA, Albany, NY, 10–11/IX/1907, E.P. Felt, Felt # a1655, deposited in Felt Collection. R. solidaginis : 3 males, 4 females, USA, West Dryden, NY, 28–30/V/1987, M.V. McEvoy; 4 males, 3 females, USA, West Dryden, NY, 15–18/IX/ 1987, M.V. McEvoy; 2 pupae USA, PA, Bucknell University Chillisquaque Creek Natural Area, 5/V/2006, N. Dorchin, ex. Solidago rugosa ; 3 larvae, USA, PA, Bucknell University Chillisquaque Creek Natural Area, 25/VIII/2006, N. Dorchin; 2 pupae, USA, PA, Liberty Valley Rd., 3/IX/2006, N. Dorchin; 2 pupae 3 females, USA, PA, Bucknell University Chillisquaque Creek Natural Area, 4/IX/2006, N. Dorchin; 2 pupae, USA, PA, Bucknell University Chillisquaque Creek Natural Area, 12/IX/2006, N. Dorchin, ex. Solidago rugosa ; 2 pupae, USA, Mifflinburg, PA, 17/V/2007, N. Dorchin & M. Wise, ex. Solidago canadensis .
Host: Solidago altissima , S. canadensis , and S. rugosa
Gall and biology: This species is bivoltine and induces morphologically different bud galls in spring (April–May) and summer (August–September), which are very similar on all three host plants. The springgeneration galls ( Figs. 70, 72 View FIGURES 70 – 77 ): are inconspicuous and difficult to locate due to the minor difference between galled and normal shoots, although the growth of galled ramets is sometimes stunted and they therefore appear shorter than ungalled ramets ( Fig. 72 View FIGURES 70 – 77 ). The apical leaves of a galled ramet appear splayed, and the base of the gall from which they originate is slightly thickened. Each gall usually contains only one conical, white chamber in the middle of the apical meristem ( Fig. 76 View FIGURES 70 – 77 ), ca. 3 mm long, and containing a single larva that is usually found deep at the bottom of the chamber with its head facing downwards. Occasionally 2–3 chambers are found in the same gall and may be attached to each other longitudinally. The chamber is surrounded by approximately 10 leaves that are much shorter and thinner than normal leaves ( Fig. 70 View FIGURES 70 – 77 ). On S. altissima , these modified leaves are lighter in color, especially along their mid vein. These are surrounded in turn by a whorl of leaves of normal shape and size. Galls contained pupae already in early May, suggesting rapid development of the spring generation soon after the plants sprout. Circumstantial evidence showed that larvae that induce the spring galls hatch from eggs in the fall of the previous year and overwinter inside the rhizomes. Rhizomes that were collected in the field, cut, and planted in our research greenhouse, yielded sprouts that developed galls without being exposed to adults (M. Wise, pers. com.).
Summer generation galls ( Figs. 71, 73 View FIGURES 70 – 77 ) become apparent around mid June and reach their final size by July, while the larvae inside them are still tiny first instars at the base of the rosette leaves. White chambers that are similar to those in the spring galls appear in the gall around mid July, when the larvae molt into third instars, and each chamber contains a single larva that is found deep in the chamber, facing downwards. Each chamber is surrounded by a group of very short and narrow leaves, which in turn are surrounded by longer and wider leaves to form a distinct subunit within the gall. Usually at least 2–5 subunits are clumped together at the shoot apex to form a conspicuous rosette that is 3–5 cm in diameter ( Raman & Abrahamson 1995) ( Figs. 71, 73 View FIGURES 70 – 77 ). The rosette gall of R. capitata ( Fig. 75 View FIGURES 70 – 77 ) is superficially similar but does not contain distinct subunits and appears flatter than galls of R. solidaginis . Pupation takes place in early September, and adults emerge in September and early October. Larvae are heavily attacked by gregarious endoparasitoids.
Remarks: This is the second largest species of Rhopalomyia on goldenrods. Males can be recognized by their very typical large and robust gonopods, and females have the shortest ovipositors of all Rhopalomyia species from goldenrods (relative to the size of the 7th abdominal tergite). Adults of the spring generation are somewhat smaller and have fewer antennal flagellomeres than adults of the summer generation, but adults and pupae of the two generations are otherwise similar morphologically.
Despite the superficial similarity between the galls of R. solidaginis and R. capitata and their phylogenetic relatedness ( Stireman et al. 2005; Dorchin et al., in prep.), they can be distinguished from each other by the male genitalia, which are larger and more robust in R. solidaginis , and by the typical shape of the Y-shaped 8th tergite of the female abdomen, the arms of which are narrow posteriorly and widen anteriorly in R. solidaginis (Fig. 38) but are of the same width throughout their length in R. capitata (Fig. 29). The antennal bases of pupae in R. solidaginis form somewhat larger horns than those in R. capitata , but pupae of the two species are otherwise very similar.
Rhopalomyia albipennis View in CoL and R. carolina View in CoL were described by Felt (1908) from galls that are similar to those of R. solidaginis View in CoL and from the same host plant ( S. altissima View in CoL , referred to by Felt as S. canadensis View in CoL ). The single female representing R. carolina View in CoL has 22–23 antennal flagellomeres but is otherwise similar morphologically to females of R. solidaginis View in CoL . Similarly, the adults of R. albipennis View in CoL are indistinguishable from those of R. solidaginis View in CoL , although Felt (1908) stated that the male wings are whitish in R. albipennis View in CoL as opposed to the brownish wings of R. solidaginis View in CoL . Examination of a slide-mounted male from Felt’s type series revealed that the wing is covered by fungal mycelia which give it a very white appearance. In two other (pinned) males from that series, we found that the wings are somewhat whitish and not covered by brown microtrichia, but we consider this an accidental example which is not representative of the species. This is because the wings of all slide-mounted and pinned females in the type series of R. albipennis View in CoL , collected at similar localities and dates, are covered by dark brown microtrichia, as are those of R. solidaginis View in CoL . Indeed, in his later revision of North American Rhopalomyia, Felt (1915) View in CoL noted that the male wings are hyaline. Based on these observations, R. albipennis View in CoL and R. carolina View in CoL are synonymized here under R. solidaginis View in CoL .
| USNM |
Smithsonian Institution, National Museum of Natural History |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Rhopalomyia solidaginis ( Loew 1862 )
| Dorchin, Netta, Mcevoy, Miles V., Dowling, Todd A., Abrahamson, Warren G. & Moore, Joseph G. 2009 |
Rhopalomyia carolina
| Felt 1908: 363 |
Cecidomyia solidaginis
| Felt 1915: 246 |
| Felt 1908: 364 |
| Loew 1862: 194 |