Paralimnadia saxitalis, Timms, Brian V., 2016
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4161.4.1 |
|
publication LSID |
lsid:zoobank.org:pub:8B9BDEA7-5F2B-465C-B2A8-757B733CCCE7 |
|
DOI |
https://doi.org/10.5281/zenodo.4685638 |
|
persistent identifier |
https://treatment.plazi.org/id/03E4878E-FFED-FFD5-FF70-07E116B1F92C |
|
treatment provided by |
Plazi |
|
scientific name |
Paralimnadia saxitalis |
| status |
sp. nov. |
Paralimnadia saxitalis View in CoL n. sp.
( Figs. 3 View FIGURE 3 , 11 View FIGURE 11 , 22 View FIGURE 22 )
Etymology. The specific name uses the Latin ‘saxitalis’ meaning ‘frequenting rocks’ which refers to the rocky waterhole on Mt Kaputar and to rocky pan gnammas on Uluru, both habitats of this species.
Type material. Holotype: AM P99020, male length 5.9 mm, height 3.8 mm, New South Wales, 35 km east of Narrabri , a rockpool on Mt Kaputar in the Kaputar National Park, 30°17’S, 150°10’E, 1 December, 1988, BVT GoogleMaps . Allotype: AM P99021, female, length 5.6 mm, height 3.2 mm, collected with holotype. Paratypes: AM P99022, 2 males, 6.1 × 3.9 mm, 5.5 × 3.2 mm, 2 females 5.6 × 3.7 mm, 5.4 × 3.2 mm, collected with holotype.
Other material examined. New South Wales: 35 km east of Narrabri , a rockpool on Mt Kaputar in the Kaputar National Park, 30°17’S, 150°10’E, 1 December, 1988, BVT, 10 males, 10 females, AM P99023 GoogleMaps , P99039. Northern Territory: Uluru , rock pools on summit, 25°20’39.4’S, 131°02’32.8”E, 1 May 1952, A. Keast and AM party, 1 specimen, AM P98561, 5 specimens , P98562.
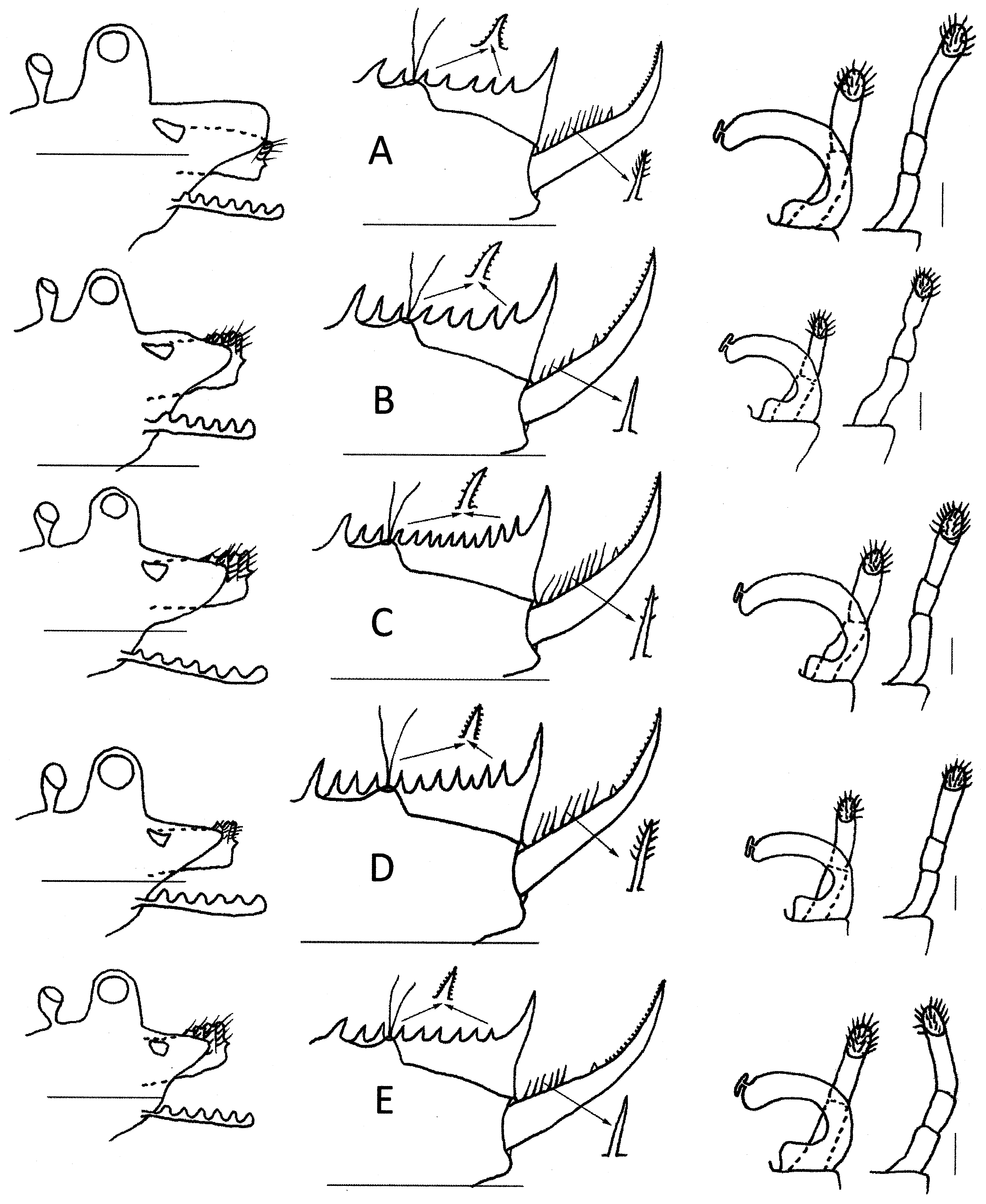
Diagnosis. Egg spherical with about 30–36 deep polygons, each with basal groove. Male rostrum broadly triangular, larger than ocular tubercle. Male clasper with long palps of 2 palpomeres, though second clasper palp partly subdivided. Telson usually with 16 ± 2 spines; cercopod usually with 9 ± 2 cercopod setae, each slightly longer than basal cercopod diameter.
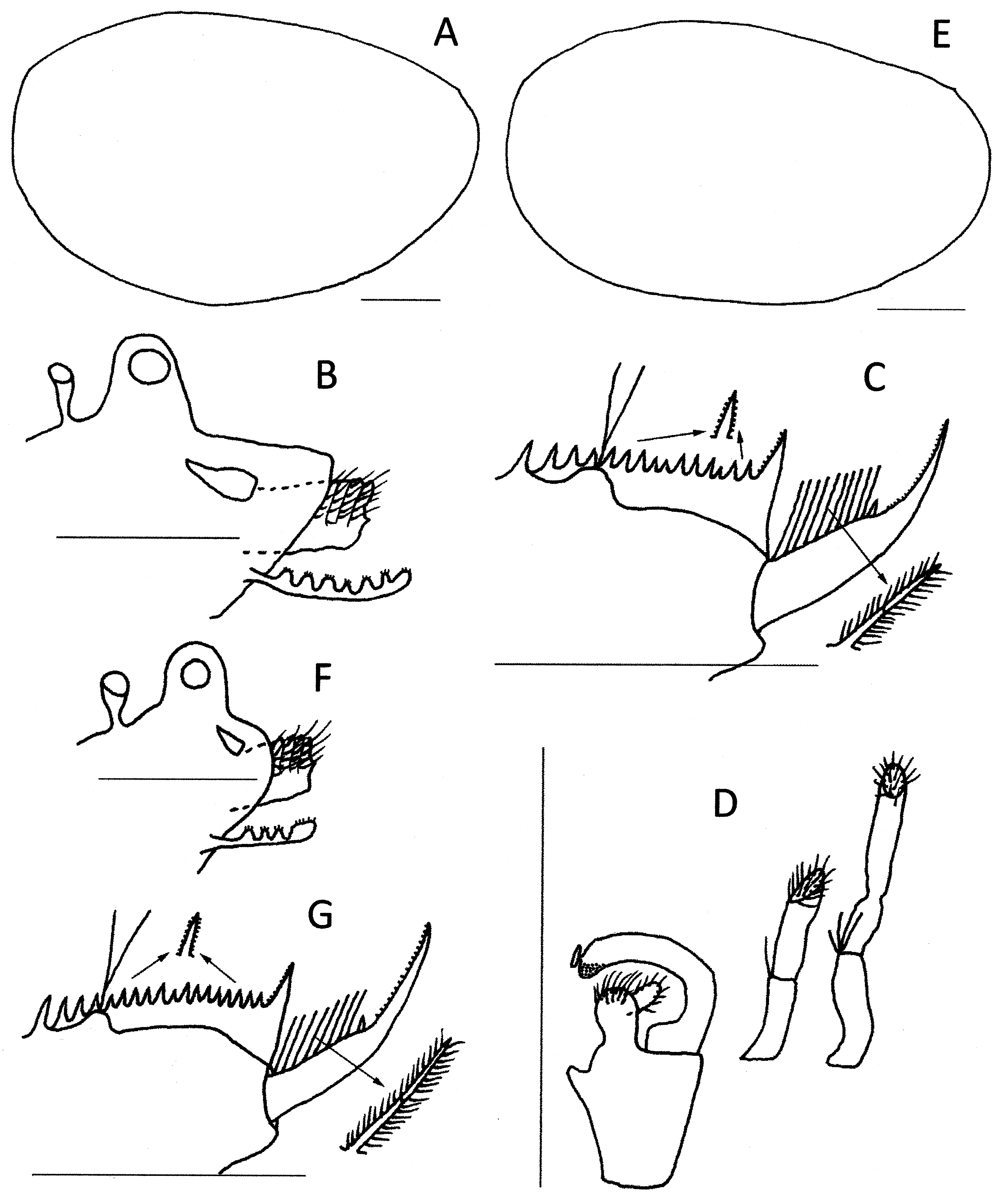
Description. Male. Head ( Fig. 22 View FIGURE 22 B) ocular tubercle prominent, compound eye occupying about 70%. Rostrum protruding about 50% more than ocular tubercle about 110° from frons, broadly triangular, apex rounded. Posterior to ocular tubercle a pyriform frontal organ about two-thirds height of ocular tubercle and separated from ocular tubercle by distance equivalent to its height. Ocellus elongated at base of rostrum, diameter slightly less than compound eye.
First antenna ( Fig. 22 View FIGURE 22 B) slightly longer than peduncle of second antenna, with about 7 sensory lobes subequal in size. Second antenna peduncle with about 12 annulations each, with spines dorsally and 2 rami; dorsal ramus with about 10 antennomeres; ventral ramus with about 11 antennomeres. Many antennomeres with 2 or 3 spines dorsally and 2–4 long setae ventrally; distally fewer spines and more setae.
Carapace ( Fig. 22 View FIGURE 22 A) pellucid with mild opaqueness ventrally, oval, almost without dorsoanterior and dorsoventral angles. Carapace dorsum weakly arched, maximum height at midlength. Abductor muscle scar at about 40° to horizontal body axis. No growth lines apparent.
Thoracopods. Eighteen trunk segments. Dorsally, trunk segments VIII–XVIII each with few short setae or spines on posterior medial edges. C laspers ( Fig. 22 View FIGURE 22 D) essentially similar in structure. Palm trapezoidal with endite III situated dorsomedially and near apical club base. Palm terminating in apical club medially and in expanded base of moveable finger laterally. Apical club longer than wide; small palp inserted medially and many long spines apically. Moveable finger arcuate, terminating in blunt apex with rounded pits ventrally. Long palp of 2 palpomeres, inserted terminally on palm; on clasper 1 palp with 2 palpomeres, with 1 or 2 spines at palpomere junction and overall 1.25 × longer than palm. Palp on clasper 2 also with 2 palpomeres, but second with narrow band and about 3 spines at palpomere junction. Distally both palps with numerous limp setae marginally on flattened palaform apical area.
Telson ( Fig. 22 View FIGURE 22 C) spine rows bearing with about 16 spines each, somewhat variable in size and spacing, with setulae laterally. Telson floor with mound at 4th spine supporting pair of setose telsonic filaments. Posterior to mound telsonic floor declivous to convex floor. Cercopod slightly shorter than telson, with cylindrical basal 55% with about 10 setae followed by short spine and finally with apical 45% narrowing to sharply concave apex and bearing cirrus of denticles. Setae geniculate, plumose, slightly longer than basal diameter of cercopod.
Female. Head ( Fig. 22 View FIGURE 22 F) largely as in male, though rostrum broadly rounded, symmetrical, and protruding less than ocular tubercle.
First antenna ( Fig. 22 View FIGURE 22 F) with about 4 unequal lobes; about as long as peduncle of second antenna. Second antenna as in male.
Carapace ( Fig. 22 View FIGURE 22 E) similar to that of male but slightly smaller.
Thoracopods. Eighteen trunk segments. Many segments with clumped dorsal setae/spines: none on segments I–IX, 3–9 on segments X–XVI and 1–3 on last two segments; setae/spines inserted on mounds along posterior portion of each segment, most noticeably on segments XII–XVII. First fifteen thoracopods all broadly similar to one another and to thoracopod III of male. None with endopodal palp. Thoracopods on segments IX and X with long epipodal filaments (flabella) to hold egg mass.
Telson ( Fig. 22 View FIGURE 22 G) as in male, but posterior spine rows with 19 spines each side and 8 cercopod setae, each subequal in length to diameter of cercopod base. Division of cercopod into setae-bearing and denticle-bearing sections closer to 50: 50 in females than males.
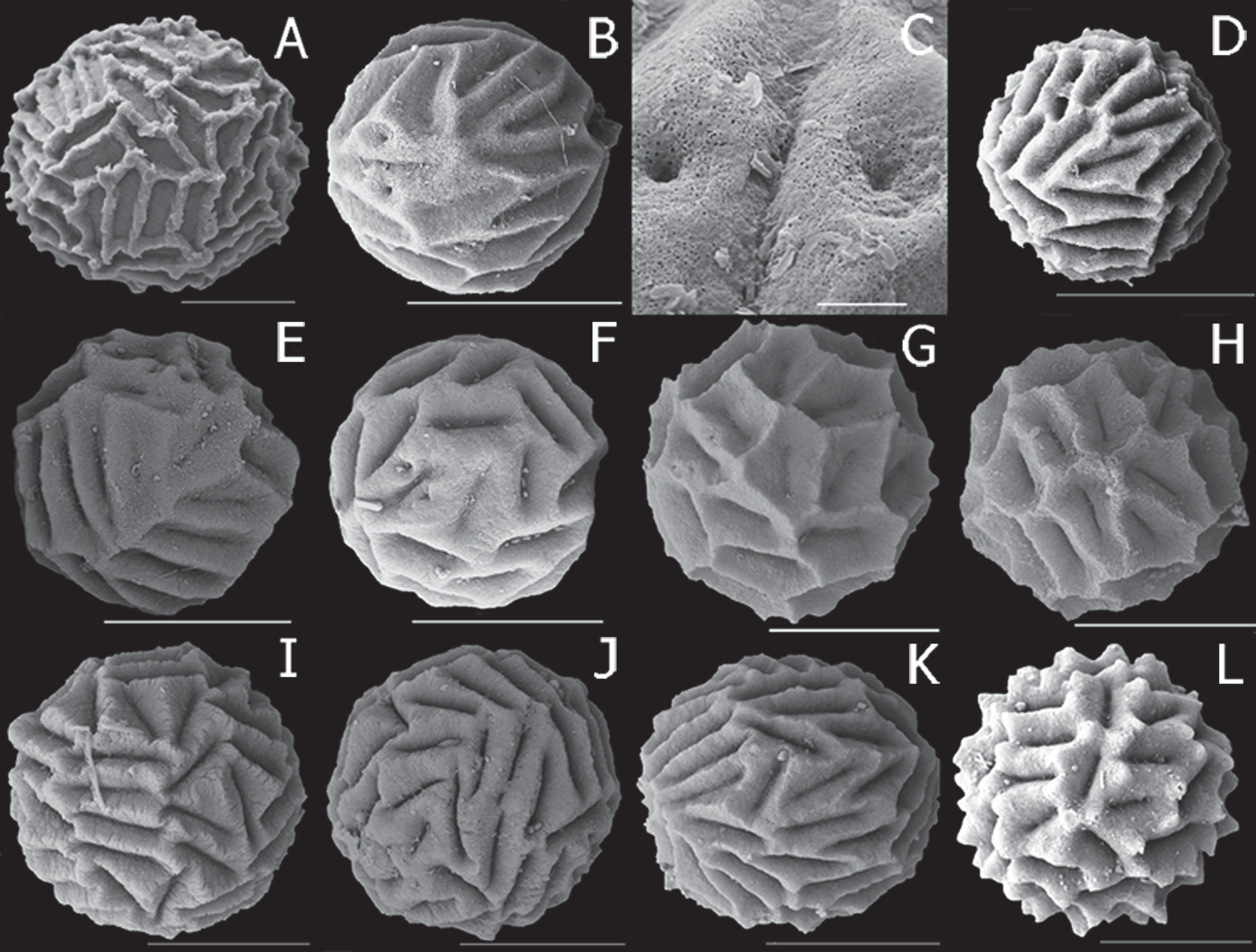
Egg ( Fig. 20 View FIGURE 20 G, H). Spherical, diameter 184 µm (range 178–195 µm, n = 5), with surface divided into 30–36 polygons (typically 4-6 sided), each with a sharp marginal ridge and steep internal slope to long groove about 30– 35 µm long.
Variability. In the Kaputar collection, some specimens have only 17 trunk segments, first antenna lobes vary ± 1, second antenna antennomeres also vary ± 1, telsonic spines range from 14–18 in males and 16–19 in females; cercopod setae range from 8–11 in males and 6–10 in females. Spines at palpomere junctions were occasionally lacking in the first large palp or reduced in the second large palp, and one specimen with the second palp of three palpomeres was encountered. Rostral size and shape and length of cercopod setae hardly varied.
The Uluru (Ayers Rock) collection contains only females. It has a similar rostrum and antennae to the Mt Kaputar material: the telson has about 19 posterior row spines and the cercopod 6 or 7 setae a little longer than the basal cercopod diameter. Cercopod proportions are slightly different, being 60% basal part with setae and 40% distal part with denticles.
Differential diagnosis. The spherical egg of P. saxilatis n. sp., with about 30–36 deep polygons is distinctive among Paralimnadia , though remarkedly similar to the egg of Southeast Asia’s Eulimnadia compressa (Baird, 1860; Rogers et al. 2016). The large (i.e., significantly bigger than the ocular tubercle) male rostrum is not unusual (some P. stanleyana , P. cygnorum , P. hyposalina n. sp., P. monaro n. sp., and P. westraliensis n. sp. also have large male rostra), but in P. saxilatis n. sp. it is triangular similar to the normal sized male rosta in other Paralimnadia . A cercopod with 7–10 setae similar in length to cercopod basal diameter occurs in P. cygnorum , P. hyposalina n. sp. and P. saxilatis n. sp, but besides rostal differences, P. saxitalis differs from P. cygnorum in having spines at basal palpomere junctionsand from P. hyposalina n. sp. in the long palp of the 1st clasper having only two palpomeres instead of three.
Distribution. Paralimnadia saxitalis n. sp. is known only two localities widely separated by nearly 2000 km, both on mountain tops, Mt Kaputar in northwestern New South Wales and Uluru in the southwestern Northern Territory. Given that there is probably no present exchange of genetic material between them, it is remarkable there is little morphological difference between them. This is the second limnadiid shrimp known from rock-top pools of Uluru, the other being Eulimnadia ulurensis (Timms 2016) . Other large branchiopods in these pools include Triops n. sp. (D.C. Rogers and author, unpublished) and Branchinella affinis and B. latzi ( Timms 2015b) .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |