Ophiotaenia lapata, Rambeloson & Ranaivoson & Chambrier, 2012
|
publication ID |
https://doi.org/ 10.5962/bhl.part.150205 |
|
publication LSID |
lsid:zoobank.org:pub:2F6506E6-C118-49FD-9ED1-A3C38C29E54F |
|
DOI |
https://doi.org/10.5281/zenodo.6303325 |
|
persistent identifier |
https://treatment.plazi.org/id/8028B07F-11C2-43B1-A798-8C5736BC72AD |
|
taxon LSID |
lsid:zoobank.org:act:8028B07F-11C2-43B1-A798-8C5736BC72AD |
|
treatment provided by |
Carolina |
|
scientific name |
Ophiotaenia lapata |
| status |
sp. nov. |
Ophiotaenia lapata View in CoL View at ENA sp. n.
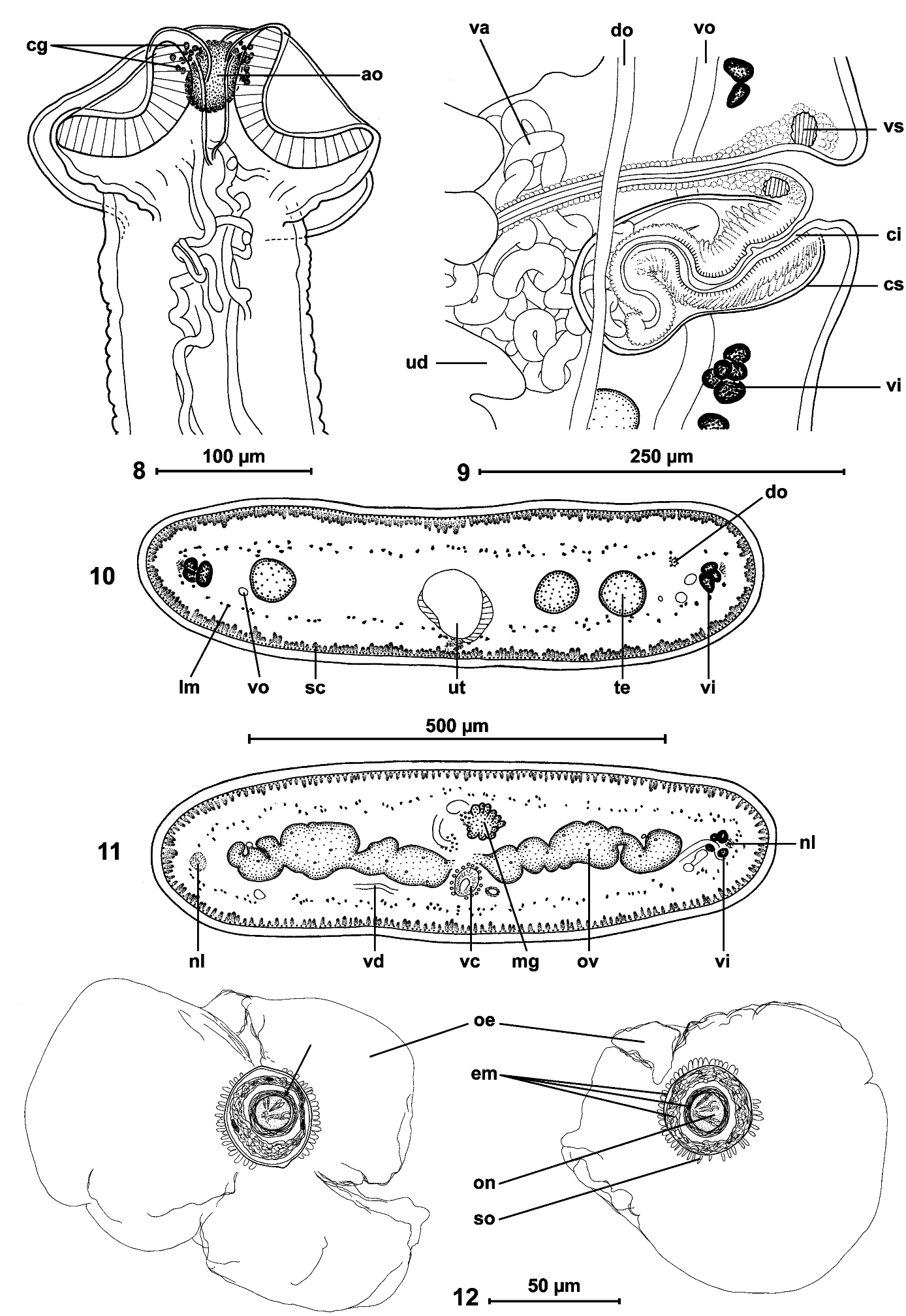
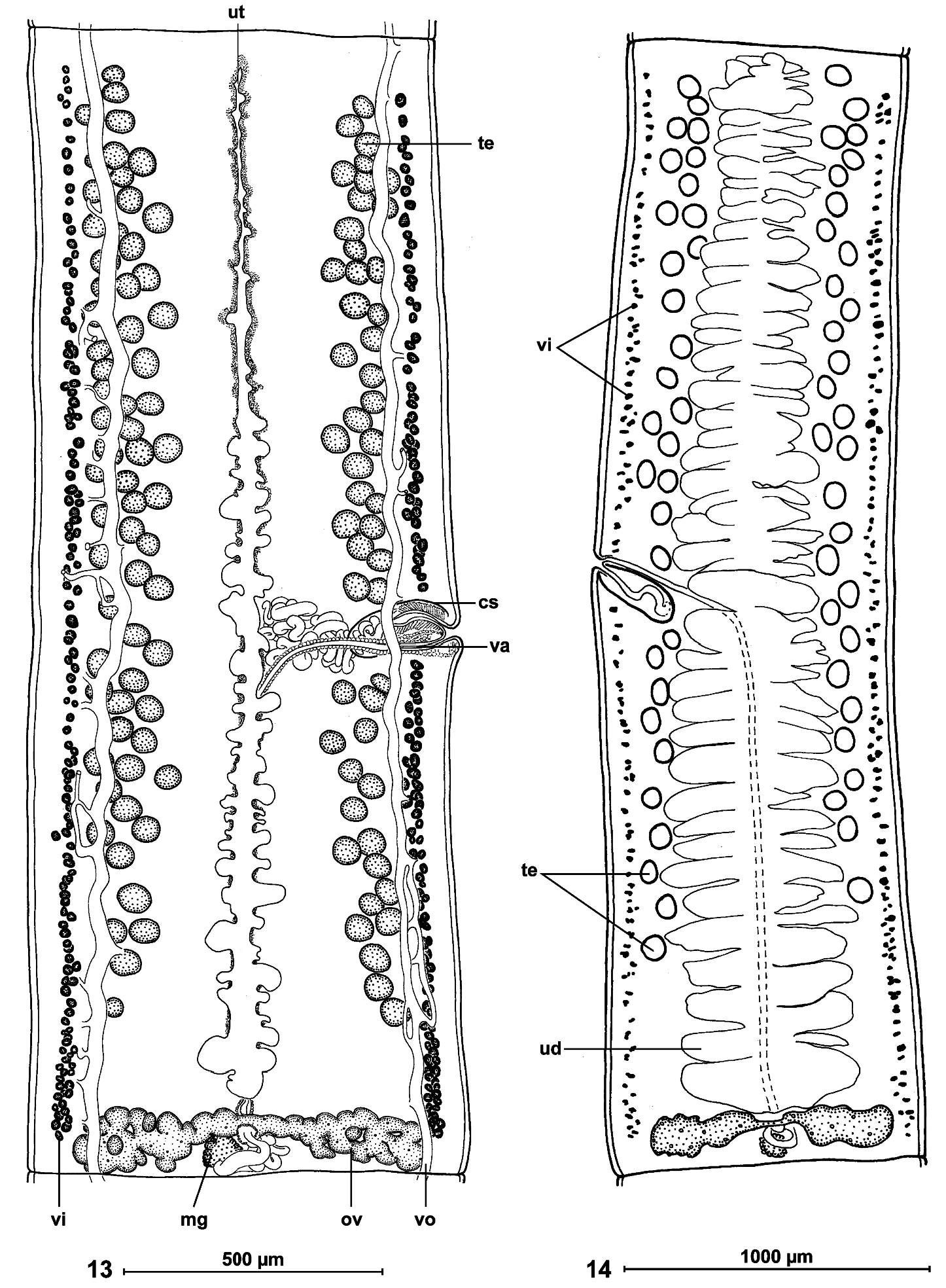
Figs 1-14 View FIGS View FIGS View FIGS
TYPE MATERIAL: Holotype MHNG-PLAT-79567 (field number Mad 007a) (1 slide) and 7 paratypes: MHNG-PLAT-79568 (Mad 007b), 3 whole mounted slides and 18 cross sections; MHNG-PLAT-82165 (Mad 007p), 1 slide; MHNG-PLAT-82166 (Mad 007x), 1 slide, scolex used for SEM; MHNG-PLAT-82167 (Mad 007z), 1 whole mounted slides and 10 transverse sections; MHNG-PLAT-79570 (Mad 008a), 1 whole mounted slide. UADBA No50001 and 50003, (Mad 007), two specimens, 2 slides.
OTHER MATERIAL: MHNG-PLAT-82172 (field number Mad 007hf), 2 whole mounted slides, (voucher material used for the study of the eggs). – MHNG-PLAT-82169 (field number Mad 007y), 1 whole mounted slides and 10 transverse sections. – UADBA No50002, 50004 (field number Mad 007), and 50005 (field number Mad 007c), 3 mounted slides. Some pieces of gravid proglottides were placed in alcohol for DNA analyses. – MHNG-PLAT-79569 (Mad 007c); MHNG-PLAT-82175 (Mad 008hf), and IPCAS C-625 , 1 whole mounted slide and 4 slides of serial cross sections (from MHNG-PLAT-82175 ). – MHNG-PLAT-73222 , from Brygoo material; Madagascar, Befandriana S. , October 1967, 1 whole mounted slide and 13 transverse sections.
TYPE LOCALITY: Ambinda Nord/Beanka (-17.93986°Lat; 44.46822°Long), 18 November 2011. All material listed above is from this locality, except MHNG-PLAT-73222.
DESCRIPTION (based on 8 specimens, 4 complete and 4 incomplete): Proteocephalidae , Proteocephalinae.
Cestodes up to 295 mm long; maximum width 1.2 mm. Strobila acraspedote, anapolytic. All proglottides longer than wide (length: width ratio 1.03-1.52 to 4.80- 6.00, from immature to gravid). Scolex 120-150 long and 190-280 (n = 3) wide, slightly wider than neck ( Figs 1-3 View FIGS , 8 View FIGS ). Suckers uniloculate, round, slightly embedded, 85- 115 (n = 12) in diameter, representing 30-48% of scolex width ( Figs 1, 2 View FIGS ). Apical organ 40-45 in diameter, i.e. 15-19% of scolex width, surrounded by cells with finely granular cytoplasm ( Fig. 8 View FIGS ). Proliferation zone 2.5-3.6 mm long and 140-185 wide.
Internal longitudinal musculature weakly developed, anastomosed, formed by numerous tiny muscle fibres ( Figs 10, 11 View FIGS ). Ventral osmoregulatory canals overlapping testes, reaching laterally vitelline follicles, 5-25 in diameter, with secondary canals directed externally; dorsal canal narrow, thick-walled, 5-10 in diameter ( Fig. 13 View FIGS ).
Testes medullary, on one layer, in two narrow lateral bands (poral field separated by terminal genitalia to preporal and postporal groups). Testes rarely reach anterior margin of proglottis, but never reach to ovary, occupying 81-88% of total length of proglottis ( Figs 10 View FIGS , 13, 14 View FIGS ). Testes 89-170 (x = 128, n = 38, CV = 17%) in number, with 47-84 (x = 66) aporal testes, 25-49 (x = 37) preporal testes and 19-38 (x = 29) postporal testes. Testes spherical, 50-65 (x = 55, n = 22) in diameter, degenerated in gravid proglottides ( Fig. 14 View FIGS ).
Cirrus-sac elongate, thick-walled, 170-250 (x = 210, n = 53) long and 75-90 (x = 80, n = 10) wide ( Fig. 9 View FIGS ); RSCS 19-26 % (x = 22%, n = 53, CV = 8 %). Cirrus length represents about 70% of cirrus-sac length. Vas deferens strongly coiled, situated between proximal part of cirrus-sac and midline of proglottides, but never crossing it.
Genital atrium present; genital pores alternating irregularly, more or less equatorial, PGP = 43-53% (x = 49 %, n = 12, CV = 6%) ( Figs 13, 14 View FIGS ). Genital ducts passing between osmoregulatory canals.
Ovary medullary, bilobed ( Figs 11 View FIGS , 13, 14 View FIGS ), 520-840 wide, RSO = 68-81% (x = 75%; n = 55; CV = 4%). Mehlis’ glands 50-80 (x = 60, n = 9) in diameter, representing 9-12% of proglottis width ( Figs 11 View FIGS , 13, 14 View FIGS ).
Vitelline follicles medullary, arranged in two lateral fields near margins of proglottides, occupying 90-95% of proglottis length, interrupted at level of cirrus-sac ( Figs 13, 14 View FIGS ).
Vaginal canal forming small seminal receptacle anterodorsal to ovarian isthmus. Terminal part of vaginal canal (pars copulathrix vaginae) surrounded by large vaginal sphincter and chromophilic cells ( Fig. 9 View FIGS ). Vagina anterior (62%; n = 26) or posterior (38%) to cirrus-sac.
Primordium of uterine stem medullary, present in immature proglottides. Development of uterus of type 1 according to de Chambrier et al. (2004): in immature proglottides, uterine stem straight, occupying most length of proglottis but never crossing ovarian isthmus, formed by wide longitudinal band of chromophilic cells situated along midline of proglottides. Lumen of uterus appearing in first mature proglottides ( Fig. 13 View FIGS ); diverticula (lateral branches) formed before first eggs appear in uterine stem. In pregravid proglottides, uterus occupying up to 33% of proglottis width, with 41-68 thin-walled lateral diverticula on each side. In gravid proglottides, diverticula occupying up to 80% of proglottis width. Uteroduct enters uterus almost at level of ovary isthmus.
Eggs round, with outer envelope 140-165 in diameter ( Fig. 12 View FIGS ). Embryophore spherical, with thick supplementary spherical layer between outer envelope and oncosphere, thus forming three-layered embryophore: internal layer 18-20 (n = 8) in diameter, middle layer 29-33 (n = 7) in diameter; external layer 34-39 (n = 8) in diameter; External layer of embryophore covered by small outgrowths 2.5-4 long; oncosphere spherical, 14-15 in diameter (n = 8), with three pairs of hooks, 8-9 long ( Fig. 12 View FIGS ). Eggs mature very fast in uterus and ripe eggs (oncospheres with hooklets) are present in the first pregravid proglottides.
TYPE HOST: Madagascarophis colubrinus (Schlegel, 1837) (Serpentes, Lamprophiidae ).
SITE OF INFECTION: Intestine.
PREVALENCE: 2/2 (100%).
ETYMOLOGY: The new species is named after the vernacular local name of the host, i.e. “lapata”.
DIFFERENTIAL DIAGNOSIS: The new species is placed in Ophiotaenia La Rue, 1911 (Proteocephalinae) because of the medullary position of the vitelline follicles, the unarmed scolex with uniloculate suckers and testes forming two separate fields ( Schmidt, 1986). Ninety-six species of Ophiotaenia parasitizing reptiles and amphibians are currently recognized as valid ( Freze, 1965; Schmidt, 1986; Ammann & de Chambrier, 2008; Marsella & de Chambrier, 2008; Coquille & de Chambrier, 2008; de Chambrier & de Chambrier, 2010; de Chambrier et al., 2010, 2012). Out of these, 64 species are parasites of snakes ( Squamata ) (see Table 1 View TABLE in de Chambrier et al., 2010).
According to Freze (1965), the species of Ophiotaenia are limited in their distribution to individual continents and/or zoogeographical regions; this assumption has been then supported by other data, like an high degree of isolation determined by the presence of a number of endemic genera such as Marsypocephalus, Sandonella (both Africa), and Goezeella (South America) for fish parasites and Rostellotaenia (Africa), Acanthotaenia (Asia) and Kapsulotaenia ( Australia, Papua New Guinea) for reptiles parasites ( Freze, 1965); furthermore, for species of Ophiotaenia from amphibian hosts ( de Chambrier et al., 2006) and from reptilian hosts ( Ammann & de Chambrier, 2008), a strict specificity (oioxenous sensu Euzet & Combes, 1980) was observed in all species of this genus. For this reason, the new species is separable from 14 Ophiotaenia species found in snakes in Africa (for their complete list, see de Chambrier et al., 2010).
Ophiotaenia lapata sp. n. differs from all but one Ophiotaenia species parasitic in African snakes by the presence of an apical organ ( Table 1 View TABLE ), the only African species possessing an apical organ being O. adiposa Rudin, 1917 described from Bitis arietans from Cameroun. Ophiotaenia lapata differs from O. adiposa by its lower number of testes (89-170 versus 170-220), position of the genital pore (situated at 43- 53% of the proglottis length from the anterior margin, i.e. almost equatorial in O. lapata , versus markedly pre-equatorial, i.e. at 20-25% length of the proglottis in O. adiposa ) and smaller scolex (width 240-280 µm in the former species versus 500-600 µm in O. adiposa ) ( Table 1 View TABLE ).
Ophiotaenia lapata n. sp. also differs from all but one Ophiotaenia species parasitic in African snakes in the possession of a third layer of the egg embryophore ( Fig. 12 View FIGS ). This layer is external to the oncosphere, i.e. it forms the internal envelope of the embryophore. The eggs of all African taxa described until now possess only a twolayered embryophore ( Beddard, 1913; Rudin, 1917; Fuhrmann, 1924; Sandground, 1928; Hilmy, 1936; Mettrick, 1960, 1963; Southwell & Lake, 1939; Freze, 1965). A similar structure, i.e. an additional layer of the embryophore, was first observed in some other Proteocephalidea tapeworms (see de Chambrier & Vaucher, 1999; de Chambrier, 2006; Coquille & de Chambrier, 2008; Marsella & de Chambrier, 2008; de Chambrier et al., 2010; de Chambrier & de Chambrier, 2010; de Chambrier et al., 2012).
This character is present in a wide range of proteocephalidean genera and geographical areas, such as Proteocephalus ( P. hobergi de Chambrier & Vaucher, 1999 ) in Paraguay, Kapsulotaenia ( K. sandgroundi Carter, 1943 ) in Indonesia, Cairaella ( C. henrii Coquille & de Chambrier, 2008 ) in Ecuador, Ophiotaenia ( O. alessandrae Marsella & de Chambrier, 2008 in Ecuador, O. gallardi (Johnston, 1911) in Australia and O. bungari de Chambrier, Binh & Scholz, 2012 in Vietnam). This additional layer of the embryophore, even if it seems to be a convergence phenomenon, is considered as a good discriminant character.
The only African species, the embryophore of which is also three-layered as in the eggs of O. lapata , is O. georgievi de Chambrier, Ammann & Scholz, 2010 described recently from Leioheterodon geayi Mocquard . This species differs from O. lapata , besides being devoid of an apical organ (see above and Table 1 View TABLE ), by the number of uterine branches (23-28 in O. georgievi versus 41-68 in O. lapata ), and by the total length of the strobila (50 mm in O. georgievi versus 295 mm in O. lapata ).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |