Nasusbilharzia melancorhypha, Flores & Viozzi & Casalins & Loker & Brant, 2021
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4948.3.5 |
|
DOI |
https://doi.org/10.5281/zenodo.4669923 |
|
persistent identifier |
https://treatment.plazi.org/id/97402C62-FFC8-FFA8-B293-E4AE95C2FA9D |
|
treatment provided by |
Plazi |
|
scientific name |
Nasusbilharzia melancorhypha |
| status |
sp. nov. |
Nasusbilharzia melancorhypha View in CoL n. sp.
( Figs. 4–6 View FIGURE 4 View FIGURE 5 View FIGURE 6 )
Type host: black necked swan Cygnus melancoryphus (Molina) .
Type locality: Mari Menuco Lake (38°35′S; 68°32′W) Neuquén Province GoogleMaps .
Other locality: Pellegrini Lake (38°41′S, 68°01′W) Río Negro Province GoogleMaps .
Prevalence of infection: 3 of 3 black-necked swans (100%) were infected in Mari Menuco Lake; 2 of 2 swans (100%) were infected in Pellegrini Lake.
Site of infection: nasal tissue
Etymology: The species name refers to the specific epithet of the definitive host.
Type material: Holotype 737, and 15 paratypes 738/1–14 deposited in MACN-Pa. Four paratypes No. 244/1–3 deposited in UNCo-Pa.
Deposition of the nucleotide sequences from this paper: MW 000330 View Materials - MW 000331 View Materials ; MW 012493 View Materials - MW 012494 View Materials .
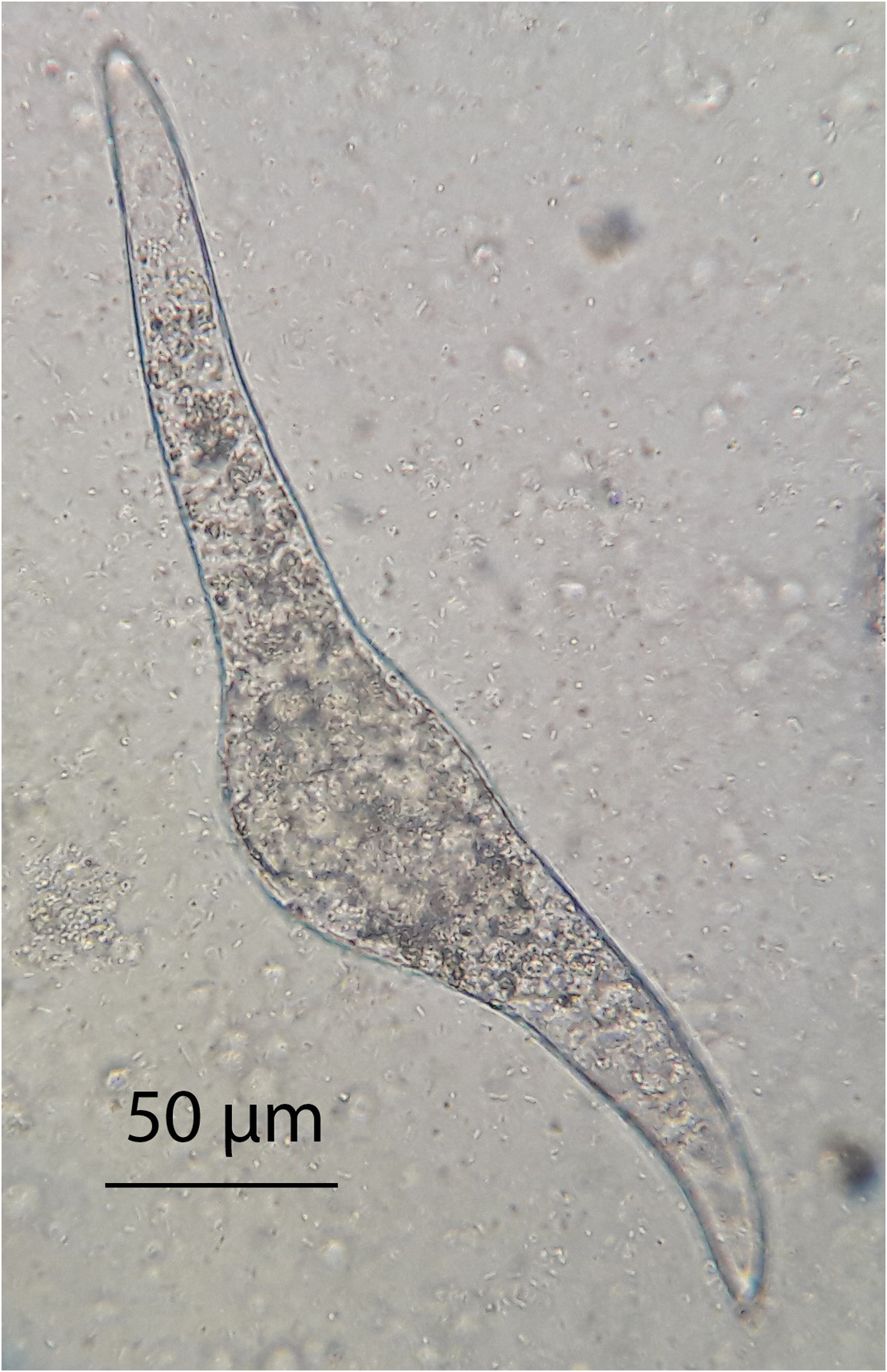
Description: Based on 20 eggs obtained from both swans of Lake Pellegrini. Eggs ( Fig. 4 View FIGURE 4 ) elongate with an asymmetrical bulge, one end with a slender process either straight or slightly curved, and the opposite end a longer curved process; 215–291 (252.6) long by 34–56 (47.9) wide.
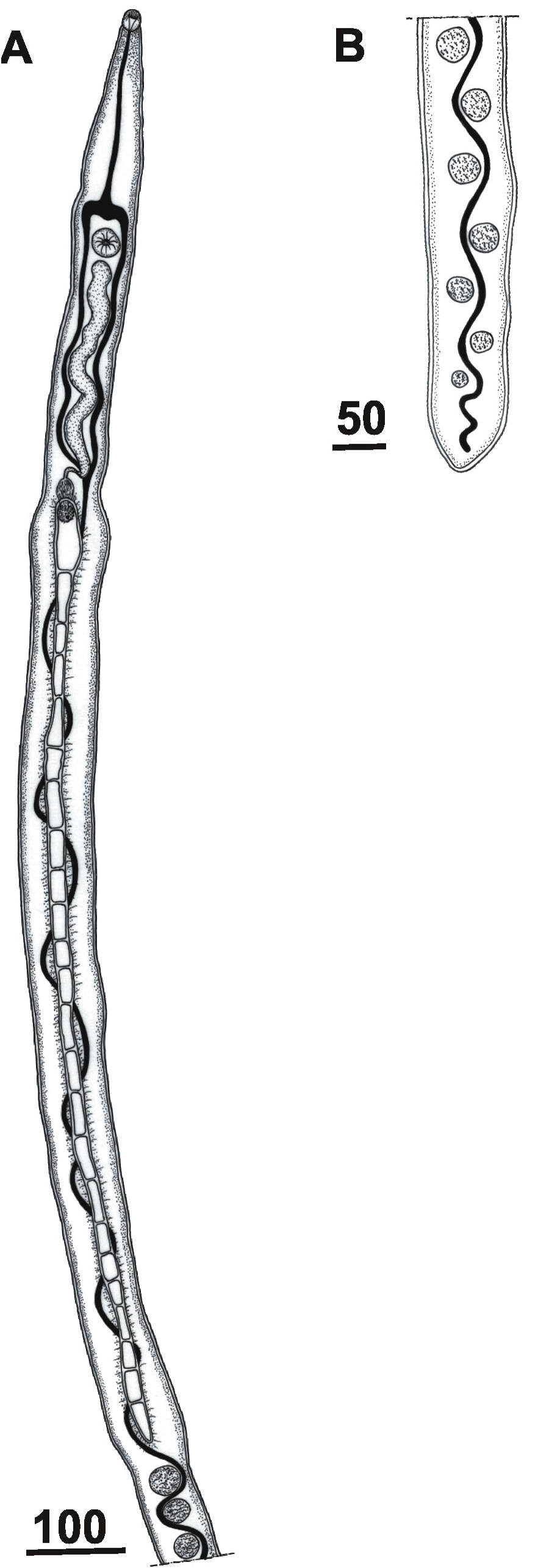
Based on 1 complete adult, and 18 anterior pieces of males recovered from nasal tissue ( Figs. 5 View FIGURE 5 , 6 View FIGURE 6 ). Male long filamentous, somewhat flattened dorso-ventrally, almost uniform width. Posterior end of body with blunt termination, not spatulated nor wider than any other part of the body. Tegument with spines. One complete adult 7,250 long, pieces between 2,414 –10,224 (5,330.3) long, wide at ventral sucker level 48–84 (63.1), at gynaecophoric canal 72–108 (82.5), posterior to gynaecophoric canal 48–84 (57.3). Oral sucker terminal 24–34 (27.7) long by 22–36 (25.8) wide. Esophagus bifurcated anterior to ventral sucker, caecal reunion at level of cirrus sac; reunited intestine continues posteriorly in a sinuous course between testes. Ventral sucker well-developed 26–48 (36.4) long by 24–43 (35.7) wide, ratio ventral to oral sucker length 1: 0.9–1.5 (1.3). Gynaecophoric canal well-developed posterior to ventral sucker, starting at 461–749 (573.7) from anterior end. Gynaecophoric canal is a spiny ventral groove 730–2,160 (1,334.3) long; divided by 17–20 (18) thickened bands. Thickened bands wide 5–7.5 (5.6). Testes spherical or slightly oval, arranged between gynaecophoric canal and posterior end of worm, numerous around 130; 25–45 (31.8) long by 25–45 (35.9) wide. Vesicula seminalis externa contorted starting at 5–24 (14) from ventral sucker, winding to end in cirrus sac, 168–312 (258.5) long by 19–43 (34.1) wide. Cirrus sac present, containing armed cirrus 31–48 (39.3) long. Ejaculatory duct ends at a well-developed cirrus. Armed cirrus located at beginning of gynaecophoric canal, 24–43 (34.6) in diameter. Genital pore at anterior end of gynaecophoric canal.
Remarks: The male specimens of Nasusbilharzia melancorhypha n. gen.; n. sp. obtained from the nasal tissues of the black-necked swan have a particular combination of characteristics that make them unique, and do not match with any previous diagnosis of schistosome genera. These worms have a filiform body with spiny tegument, a blunt posterior end, two well developed muscular suckers, a robust gynaecophoric canal with thickened cross bands, armed cirrus, and testes start at the posterior end of the gynaecophoric canal with around 130 testes. Results of the sequence analysis confirm the distinctiveness of these worms and place them at the base of the large avian schistosome clade. They do not group with any of the more commonly found genera from anatids like Allobilharzia Kolářová, Rudolfová, Hampl, & Skírnisson, 2006 , Anserobilharzia Brant, Jouet, Ferte, & Loker, 2013 , and Trichobilharzia ( Figure 2 View FIGURE 2 ; Table 1 View TABLE 1 ). Additionally, relatively few species of Trichobilharzia inhabit the nasal tissue as adults ( Table 2 View TABLE 2 , 3).
GC = Gynaecophoric canal
Nasusbilharzia is similar to Allobilharzia and Anserobilharzia in the development of suckers ( Kolářová et al. 2006; Brant et al. 2013), but they differ by having a short gynaecophoric canal in relation to body length, and by having testes that start some distance posterior to the posterior end of the gynaecophoric canal ( Table 1 View TABLE 1 ). Nasusbilharzia resembles Gigantobilharzia Odhner, 1910 in having an armed cirrus, a gynaecophoric canal with thickened bands and testes starting at the posterior end of gynaecophoric canal but Gigantobilharzia differs by having weakly developed suckers and a broadened body end ( Khalil 2002). The new species differs considerably from Dendritobilharzia Skrjabin & Zakharov, 1920 since the latter genus has a shorter gynaecophoric canal and underdeveloped suckers and a cirrus without spines ( Table 1 View TABLE 1 ). The new species is similar to the nasal Trichobilharzia species ( T. rodhaini Fain, 1955 , T. nasicola Fain, 1955 , T. spinulata Fain, 1956 , T. aureliani Fain, 1956 , T. duboisi Fain, 1959 , T. australis Blair et Islam, 1983 , T. arcuata Islam, 1986 , T. regenti ) in having well-developed suckers, but these Trichobilharzia differ by having a broadened posterior end, and a much shorter gynaecophoric canal without thickened bands ( Fain 1955a; 1955b; 1956; 1959; Blair & Islam 1983; Islam 1986, Hórak et al. 1998) ( Table 2 View TABLE 2 ). In addition, the species described herein differs in having a much longer gynaecophoric canal with at least 17 thickened bands. The infection site is also considered an important taxonomical feature since nasal-inhabiting specimens are not conspecific with specimens in the visceral blood vessels, as it was pointed out for Trichobilharzia species ( Blair & Islam 1983).
According to Blair & Islam (1983) almost all Trichobilharzia species produce approximately the same shape of egg. The eggs obtained from nasal tissue of black necked swans are similar in shape to those of Trichobilharzia species from nasal tissues; there is a middle bulge, with one end with a straight to slightly curved process and the opposite end a longer curved one. The egg size of Nasusbilharzia melancorhypha is within the range given for other species that parasite nasal tissue ( Table 3). Nasusbilharzia melancorhypha eggs differ from those of the genus Giganthobilharzia which have oval eggs with a spine on one end ( Akramova et al. 2010); they differ from Dendritobilharzia eggs which are round to oval, and aspinose ( Vande Vusse 1980); they differ from those of Allobilharzia , which have a “duck head” shape at one end, and at the other end, a slender prolongation with a slightly curved process at the top ( Kolářová et al. 2006); and they differ from Anseroblharzia eggs which are ovoid and have a small recurved terminal spine ( Brant et al. 2013).
Ref. = Reference (1) Fain, 1955a; (2) Fain 1956; (3) Fain, 1959; (4) Blair and Islam, 1983; (5) Islam, 1986; (6) Horák et al.
1998; (–) = no data available
TABLE 3. Morphological data for eggs of nasal schistosome of different genera and for Nasusbilharzia melancorhypha parasitizing aquatic birds.
| Species | Ref. | Bird Host | Country | Length | Width |
|---|---|---|---|---|---|
| Nasusbilharzia melancorhypha | this study | Cygnus melancorphya | Argentina | 215–291 | 34–56 |
| Trichobilharzia rodhaini | 1 | Hagedashia hagedash | Congo Rwanda | 280–550 | 70 |
| Trichobilharzia nasicola | 1 | Anas undulata | Congo Rwanda | 280–330 | 50–70 |
| Trichobilharzia aureliani | 2 | Pocliceps cristatus infuscatus | Congo Rwanda | 250–280 | 50–70 |
| Trichobilharzia spinulata | 2 | Plectopterus gambensis | Congo Rwanda | (–) | (–) |
| Trichobilharzia duboisi | 3 | Nettapus auritus | Congo Rwanda | 282–330 | 52–70 |
| Trichobilharzia australis | 4 | Anas supersiliosa | Australia | 254–288 | 56 |
| Trichobilharzia arcuata (experimental) | 5 | Dendrocygna arcuata | Australia | 97–153 | 47–63 |
| Trichobilharzia regenti (experimental) | 6 | Anas platyrhynchos , Cairina moschata | Germany | 260–318 | 76–102 |
| MW |
Museum Wasmann |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |