Melanosternarchus amaru, Bernt & Crampton & Orfinger & Albert, 2018
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4378.4.1 |
|
publication LSID |
lsid:zoobank.org:pub:B9182B25-7988-4020-92AF-3B6EE016373E |
|
DOI |
https://doi.org/10.5281/zenodo.6490623 |
|
persistent identifier |
https://treatment.plazi.org/id/03AF5047-FFA0-A335-FF77-FD5284415D43 |
|
treatment provided by |
Plazi |
|
scientific name |
Melanosternarchus amaru |
| status |
sp. nov. |
Melanosternarchus amaru , new species
Holotype. MUSM 59405 , 230 mm TL, Peru, Loreto, Maynas, Rio Nanay at Pampachica , 03°45'10.8"S, 73°16'54.2"W, M. Bernt, A. Chota et al., 2 January 2016. GoogleMaps
Paratypes. Peru: Loreto: ANSP 200451 View Materials (1), 262 mm TL and ANSP 200452 View Materials (1), 177 mm TL, Rio Nanay downstream from Pampachica , 03°43'26.1"S 73°17'11.6"W, M. Bernt et al. GoogleMaps , 4 July 2014. ANSP 200459 View Materials (12, 2 cleared and stained), 149–231, collected with holotype GoogleMaps .
Nontypes. Brazil: Amazonas: ANSP 192675 View Materials (1), 160 mm TL , Rio Negro, 35 km downriver of Vila Guajará, 36 km upriver of Carvoeiro , 01°13'23'' S 62°14'0''W, J. Lundberg et al., 11 December 1993 GoogleMaps . ANSP 192676 View Materials (3), 165–250 mm TL, Rio Negro, 33.9 km downriver of Novo Caioe, 6.5 km upriver of S. Francisco de Assis, J. Lundberg et al., 5 December 1993 . FMNH 114883 View Materials (2), 138–149 mm TL, Rio Purus, between tributaries Boca do Lago do Estopa and Solimões , between towns Surará and Beruri, 03°57'28" S 61°27'41"W, J. Lundberg et al., 27 July 1996 GoogleMaps . FMNH 128388 (2), 208–210 mm TL, FMNH 128397 View Materials (1), 236 mm TL, and FMNH 128405 View Materials (1), 271 mm TL, Rio Negro near confluence with Rio Amazonas , 03°08'24.6"S 059°54'22.8"W, V. Tagliacollo et al., 15 May 2016 GoogleMaps . ZUEC 14195 View Materials (1), 272 mm TL, Solimões-Japurá confluence at Mamirauá Lake System, Paraná Maiana 03°06'36"S 064°47 04" W, W. Crampton, 28 January 1999 . Pará: ANSP 196460 View Materials (1), 235 mm TL, Rio Xingu, right bank, ca. 1.3 km northwest (downstream) of Porto de Moz , 01°44' 40°5''S 52°14' 52°3''W, M. Arce et al., 3 April 2014 GoogleMaps . LSUMZ 20733 View Materials (1), 237 mm TL and LSUMZ 20734 View Materials (1 cleared and stained), 239 mm TL, Rio Amazonas at Tocumatuba, near mouth of small channel connecting to Rio Tapajós , 2°14'38''S 54°47'48.2''W, J. Albert et al., 19 June 2015 GoogleMaps . LSUMZ 20732 View Materials (1), 198 mm TL, Rio Amazonas at Tocumatuba, ca. mouth of Rio Tapajós , 2°13'56.2''S 54°48'24.7''W, M. Bernt et al., 21 June 2015 GoogleMaps . Peru: Loreto: MUSM 59453 (19, 2 cleared and stained), 145–242 mm TL, Rio Nanay downstream from Santa Clara , 03°45'42.72"S 073°18'54.06"W, M. Bernt et al., 5 January 2017 GoogleMaps . MUSM 59450 (4), 135–172 mm TL, Rio Nanay at Pucayacu a Orillas , 03°45'26.94"S 073°18'37.86"W, M. Bernt et al., 2 January, 2017 GoogleMaps . MUSM 56870 (4), 161–170 mm TL, Rio Nanay near Iquitos at Puerta Camelias , 03°42'47.1'' S 073°18'19.7''W, A. Orfinger et al., 23 December 2015 View Materials GoogleMaps . ZUEC 14196 (1), 195 mm TL, beach near Playa Pampachica, A. Orfinger et al., 7 September 2015.
Diagnosis. As for the genus.
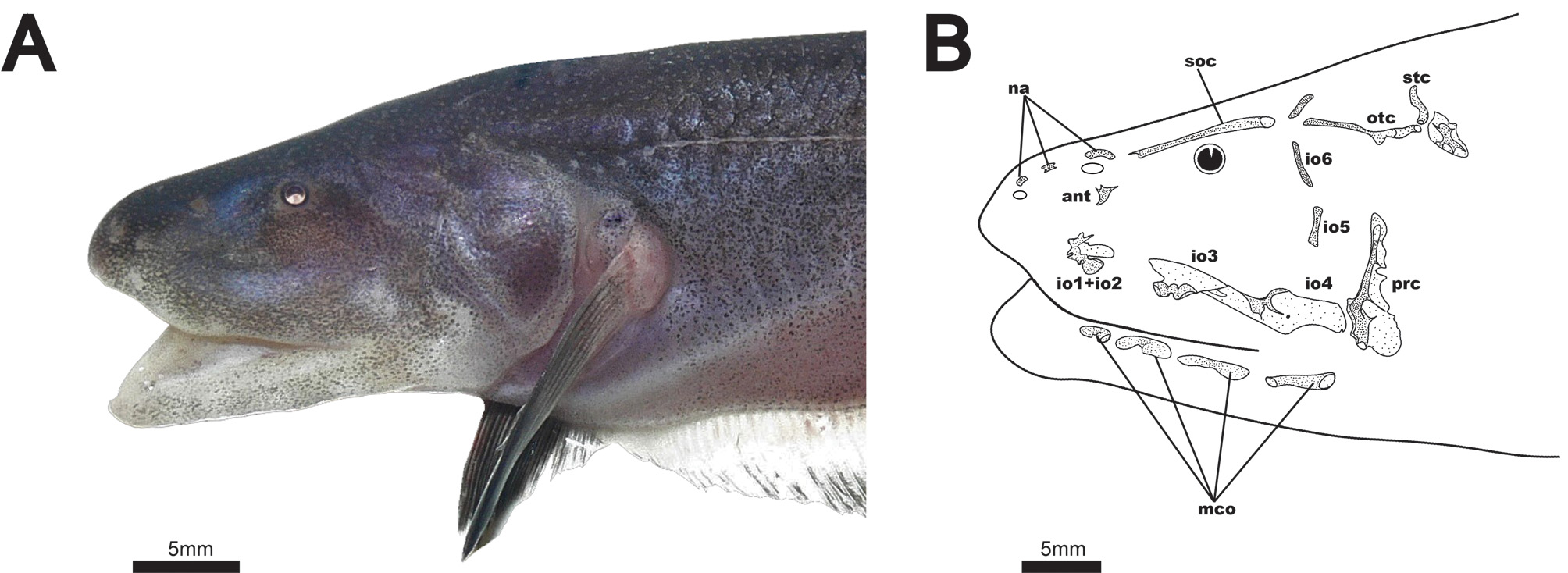
Description. Body shape and pigmentation illustrated in Figs 1–3 View FIGURE 1 View FIGURE 2 View FIGURE 3 . Summaries of morphometric data on body measurements and meristics are presented in Table 1. Largest recorded size 272 mm TL. Body laterally compressed, elongate and slender. Head and mouth large, rictus extending beyond vertical with eye. Mouth terminal or slightly inferior. Eye small, less than 10% head length, covered by thin layer of skin. Scales large and rhomboid forming 3–4 rows above lateral line at midbody. Scales absent on entire middorsum and patch over nape above lateral line to about 5th lateral-line pore. Nasal capsule closer to snout tip than to eye. Anterior nares tubular.
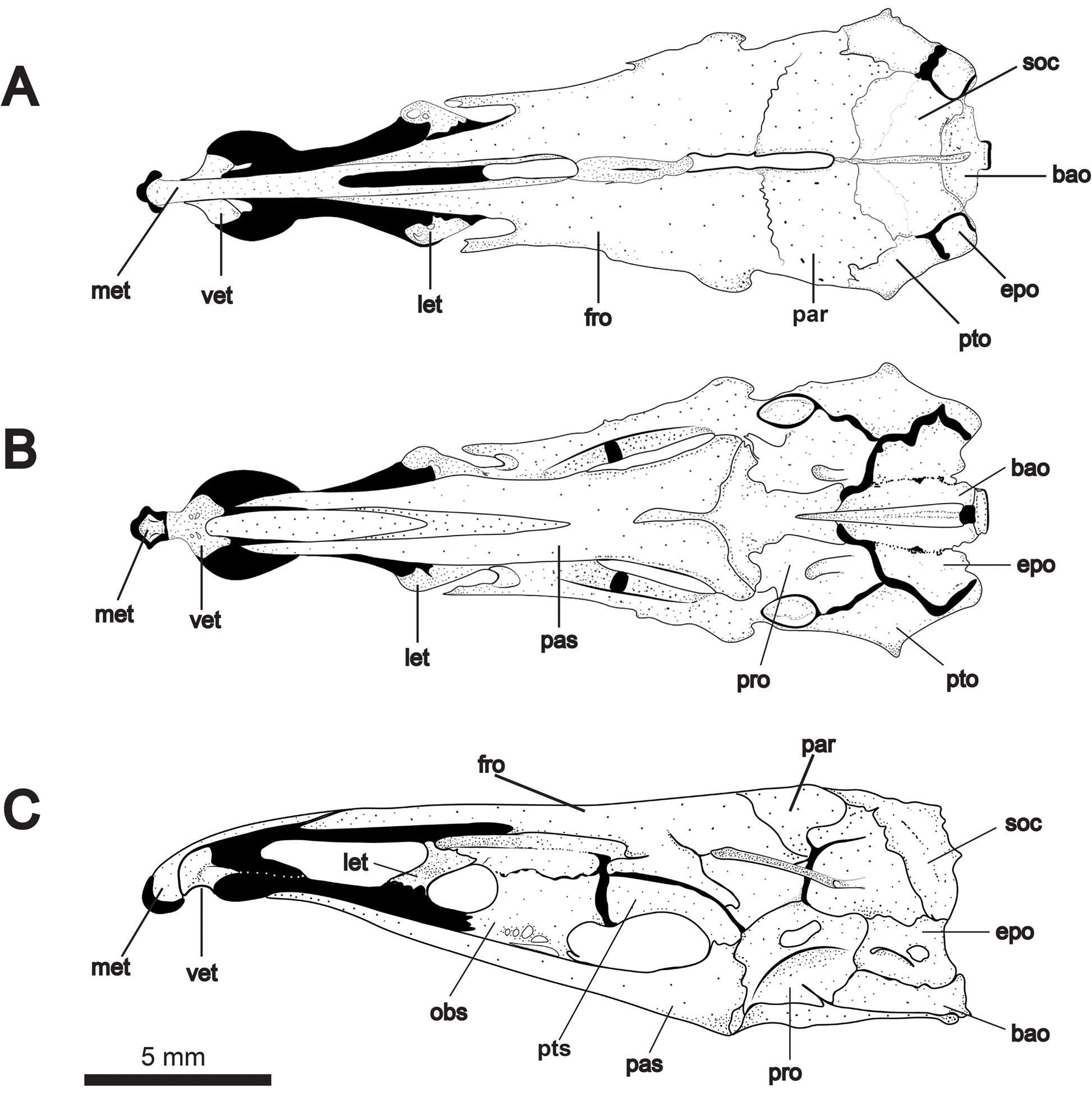
Neurocranium illustrated in Figs. 4 View FIGURE 4 and 5 View FIGURE 5 . Paired frontals straight or slightly concave in dorsal profile and comprising about half total skull length. Posterior fontanelle about one-third longer than anterior fontanelle. Supraoccipital crest not exceeding dorsal margin of parietals. Parietals surround posterior half of posterior fontanelle, and frontals surround anterior half of posterior fontanelle. Mesethmoid strongly decurved and forked posteriorly, underlying nasals and bordering anterior fontanelle. Anterior tip of mesethmoid with bulbous, cartilaginous projection bearing short lateral processes that articulate with premaxillae. Ventral ethmoid robust with wing-like lateral processes contacting lateral ethmoid cartilage. Lateral ethmoids robust, broadest at dorsal articular surface and angled obliquely to neurocranial axis. Orbitosphenoid contacting frontals dorsally and parasphenoid at two points ventrally. Orbitosphenoid cartilaginous dorsally in specimens up to 200 mm TL. Orbitosphenoid contacts pterosphenoid posteriorly. Together with the parasphenoid, these bones form a prominent lateral fenestra. Margin of articulation between orbitosphenoid and pterosphenoid protrudes ventrally into anterior third of this fenestra. In one adult specimen ( Fig. 4C View FIGURE 4 ), pterosphenoid bears posterior ventral process extending to parasphenoid (also present in Orthosternarchus tamandua Boulenger, Hilton et al., 2007 : fig. 10B; Sternarchella schotti Steindachner and S. raptor Lundberg et al. , Ivanyisky & Albert, 2014: figs. 3B, F; and Compsaraia compsa Albert, Bernt & Albert, 2017 : fig. 5A). The parasphenoid is the largest element of the skull floor contacting the basioccipital and prootics posteriorly, and bifurcating anteriorly into sharply-pointed processes that reach the cartilage of the lateral ethmoids at or near its junction with the ventral ethmoid. Dorsal to the parasphenoid, the vomer tapers to a sharp point posteriorly and broadens anteriorly at its contact with the ventral ethmoid.
The braincase is composed of the basioccipital, supraoccipital and paired parietals, epiocciptals, exoccipitals, prootics, pterotics, and sphenotics. Supraoccipital crest low, not exceeding dorsal margin of parietals. Pterotics support horizontal semicircular canals (visible ventrally) and project laterally to the skull’s greatest width. Prootic and exoccipital with prominent foraminae. Basioccipital approximately triangular in ventral view, with deep medial groove and a circular patch of cartilage at its posterior base.
Suspensorium and oral jaws illustrated in Fig. 6 View FIGURE 6 . Opercle triangular with a slightly concave dorsal margin. This bone is reticulated and weakly ossified at its center, becoming more laminar at its distal margins. Interopercle teardrop shaped, more heavily ossified dorsomedially. Ventral extension is extremely thin and remains transparent after alizarin staining. Subopercle similarly thin and transparent along distal margin. Preopercle with vertically-oriented laterosensory canal tube on lateral surface. Hyomandibula oriented nearly 90° to long axis of skull, with ridge projecting posterolaterally, tightly articulating with dorsal half of preopercular anterior margin. Dorsal articulating head of hyomandibula about twice width of distal end. Prominent foramen associated with cranial nerves (V, VII, and lateral line nerves) visible on medial surface. Symplectic triangular, oblique to hyomandibula and separated by a thick band of cartilage. Metapterygoid triangular with dorsal and ventral sides about equal in length. Quadrate articulating ventroposteriorly with preopercle in two interlocking processes from each bone. Endopterygoid edentulous with a robust, vertical (i.e. perpendicular to bone’s dorsal surface) ascending process. Endopterygoid broadly overlapping with metapterygoid and quadrate (visible medially). Autopalatine cartilage extending from anterior tip of endopterygoid and abruptly curving ventrally to contact the articular surface of the maxilla.
The mandible is composed of the retroarticular, anguloarticular, dentary, coronomeckelian bones, and Meckel’s cartilage. Retroarticular small, roughly rectangular without anterior process overlapping with anguloarticular. Anguloarticular with short ventroposterior process overlapping ventral surface of retroarticular. Anteriorly, anguloarticular has a complex overlapping articulation with the dentary, most clearly seen in lateral view. Dentary with 17–24 recurved teeth at middle of dorsal (oral) margin, arranged in two irregular rows posteriorly and merging into a single row anteriorly. Teeth on inner row angled medially. Anterior 1/3 and posterior 1/4 of dentary lacking teeth. Dorsoposterior region of dentary forming short, sharply-tapering process. Meckel’s cartilage prominent on medial surface of mandible, slightly wider posteriorly than anteriorly. Dorsal to Meckel’s cartilage, coronomeckelian is visible as a slender, triangular sesamoid ossification.
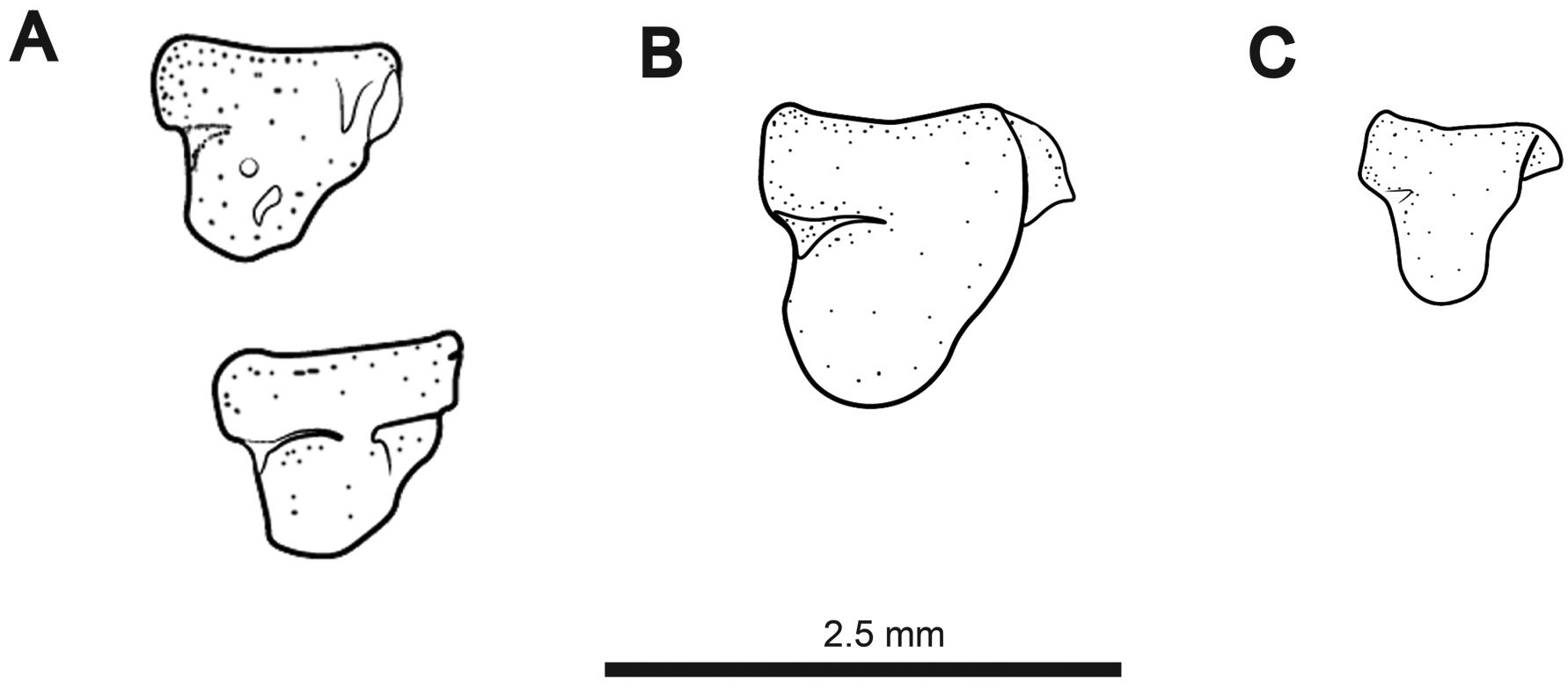
Maxilla with anteroventral shelf and anterodorsal hook. Descending blade of maxilla gracile and extending only slightly below ventral margin of anteroventral shelf. Articular surface of maxilla with rounded, cartilaginous cap. Premaxilla semicircular to triangular in ventral view. Ventral surface flat. Dorsal surface with anterior ridge and posterior depression at point of articulation with mesethmoid. Premaxilla edentulous or with a single conical tooth in anterolateral corner ( Fig. 7 View FIGURE 7 ).
The elements of the ventral hyoid arch are illustrated in Fig. 8A View FIGURE 8 . Posterior ceratohyal with prominent foramen on dorsolateral surface. Entire anterior surface of posterior ceratohyal contacting anterior ceratohyal. Expanded cartilaginous margin between ceratohyals forming prominent ventral protuberance. Dorsal hypohyal with deep dorsolateral pit or fossa. Ventral hypohyal nearly pyramidal (triangular in lateral view), with all sides of approximately equal length. Urohyal ( Fig. 8B View FIGURE 8 ) without posterior blade. Dorsal surface of urohyal with medial ridge extending to 3/4 of bone’s length. Ventral surface with only a very short medial eminence. Four branchiostegals. Branchiostegal 1–3 contact the ventral surface of the anterior ceratohyal. Branchiostegal 1 and 2 slender and filamentous, broadest at dorsal end. Third branchiostegal scythe-shaped with thin, poorly-ossified ventral margin. Branchiostegal 4 axe-shaped with long, slender posterior process, thin, weakly-ossified bladelike descending process, and short, pointed anterior process.
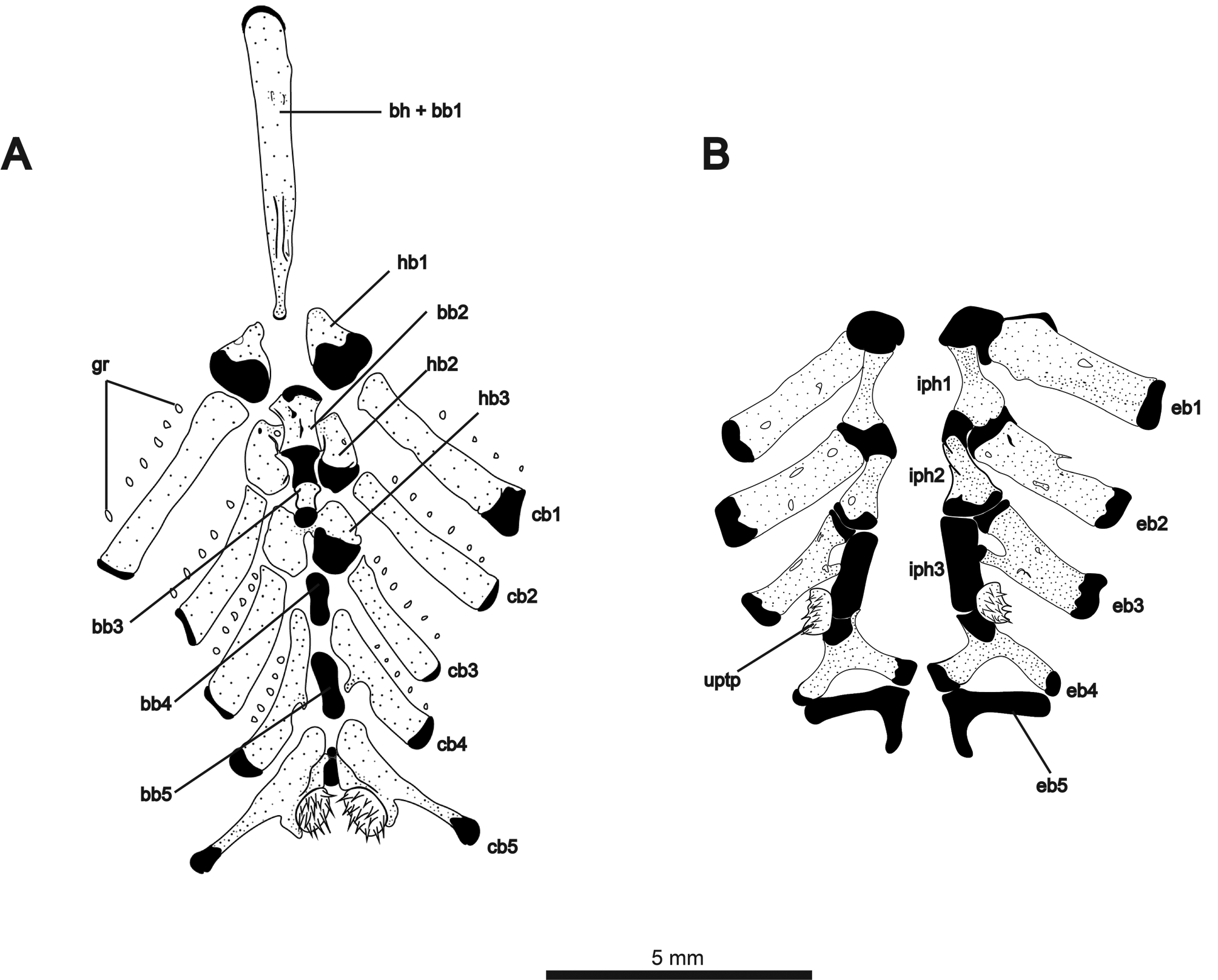
The ventral components of the branchial basket are illustrated in Fig. 9A View FIGURE 9 . Gill rakers ossified, but not contacting gill-arch bars. Basihyal with cartilaginous anterior tip and short ridge on posterodorsal surface. Second basibranchial hourglass-shaped, convex at anterior and posterior articular surfaces. Third basibranchial ossified, broader anteriorly than posteriorly. Basibranchials 4 and 5 unossified. First hypobranchials triangular and cartilaginous posteriorly. Second hypobranchials with anteromedial contact beneath second basibranchial. Third hypobranchials contacting medially posterior to third basibranchial. Ceratobranchials 1–3 roughly rectangular, with cartilaginous cap at dorsal tip. Ceratobranchial 4 with posteriorly-angled medial process at about midlength of bone. Four to six gill rakers associated with each of first four ceratobranchials. Fifth ceratobranchials contacting medially around a short, cylindrical element of cartilage. This piece of cartilage was tentatively identified by Hilton et al. (2007) as basibranchial 5 in Orthosternarchus tamandua (see their fig. 14). However, in Melanosternarchus , this structure lies posterior to two much larger elements that we identify as the last two basibranchials, making this structure between the fifth ceratobranchials of unknown identity. Posteromedial surface of ceratobranchial 5 bearing 13–15 sharply-pointed, conical teeth. Dorsal elements of the branchial basket illustrated in Fig. 9B View FIGURE 9 . Epibranchials 1–3 approximately rectangular, associated with few (1–2) or no gill rakers. Epibranchial 3 with short anteromedial process contacting infrapharyngobranchial 3. Epibranchial 4 Y-shaped with elongate posteriorly- and medially-oriented processes, both contacting epibranchial 5. Epibranchial 5 L-shaped and entirely cartilaginous. Infrapharyngobranchials 1 and 2 broad posteriorly with thick cartilaginous articular surface contacting epibranchial laterally and next infrapharyngobranchial medially. Infrapharyngobranchial 3 cylindrical and cartilaginous. Region of articulation between infrapharyngobranchial 3 and epibranchial 4 bearing the upper pharyngobranchial tooth plate, which holds 10–12 conical teeth.
Cranial laterosensory canals illustrated in Fig. 3B View FIGURE 3 . Three nasal canal-bones present as short, tubular ossicles. Elongate supraorbital canal fused with frontal bone. Antorbital bone present as small, triangular, laminar dermal ossification just below posterior nares. Infraorbital bones 1 and 2 fused with trifurcating anterior tubular ossifications, and a laminar dermal posterior ossification. Infraorbital 3 with ventral tubular ossification and laminar dermal projection dorsally. Infraorbital 4 with dorsal tube and a ventral laminar dermal projection extending posteriorly and anteriorly. Laminar dermal portions of infraorbitals 3 and 4 with broad, overlapping contact. These bones are not fused, however, as they readily separate in cleared and stained specimens. Infraorbitals 5 and 6 present only as thin, tubular canals. Infraorbital 5 very weakly ossified or absent in some specimens. Parietal canal independent from supraorbital, angled posteriorly. Otic canal present as a groove or incompletely-closed tube over anterior 2/3 to 3/4 length. Supratemporal canal short, not strongly angled dorsoposteriorly. Four free mandibular canals (2–5), present as distally-expanded ossicles. All ventrolateral to the mandible, with none ventral to preopercle. First mandibular canal present as foramen in anterior region of dentary ( Fig. 6 View FIGURE 6 ).
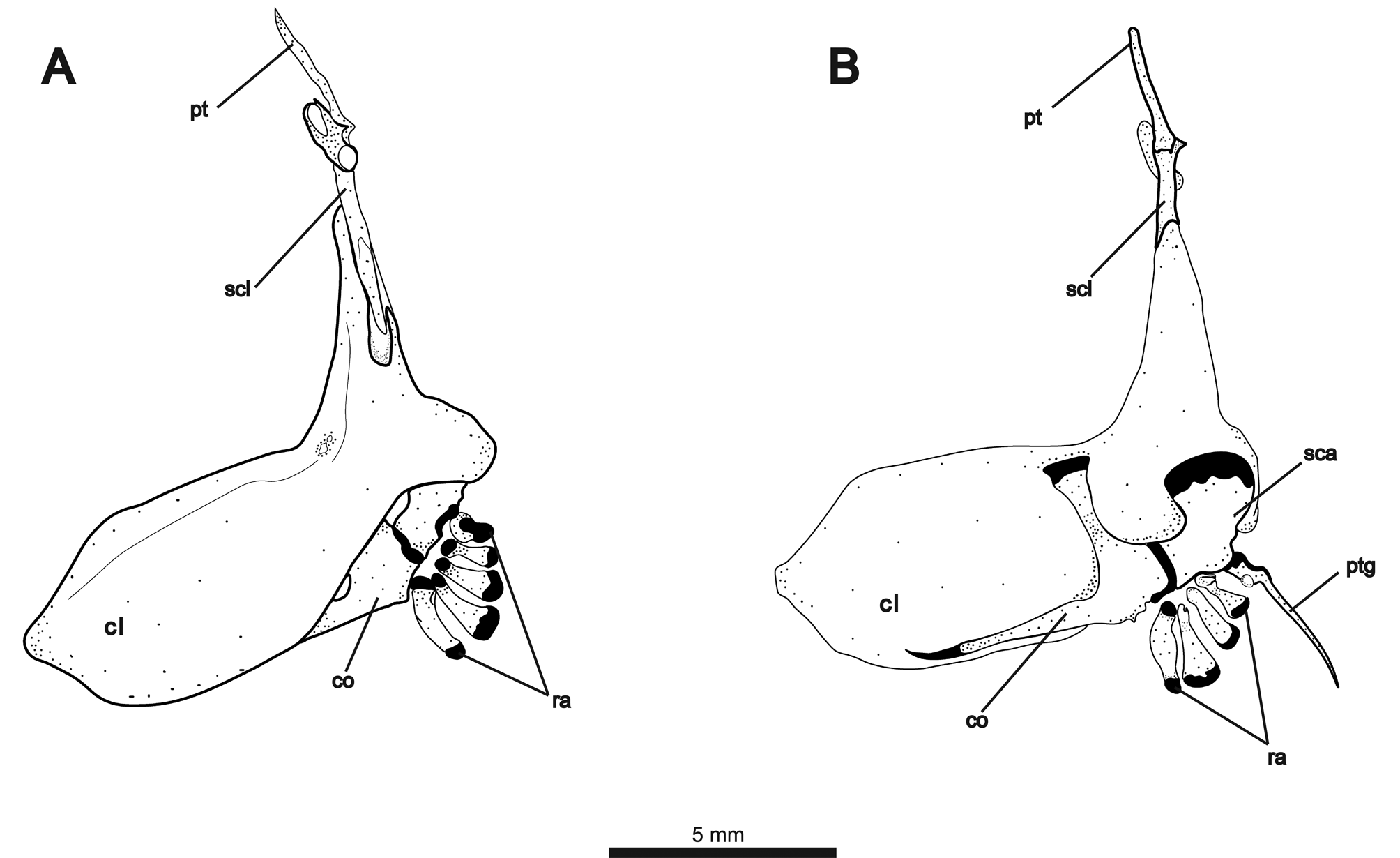
Elements of pectoral girdle illustrated in Fig. 10A View FIGURE 10 (lateral view) and 10B (medial view). Posterior edge of cleithrum curved medially, wrapping around posterior margin of scapula. Two weakly-ossified, rectangular postcleithrae present on each side. About 2/3 of supracleithrum with overlapping contact to dorsal extension of cleithrum. Dorsal tip of supracleithrum forming notch-like articular surface for ventrally-broadened posttemporal. Anterodorsally angled laterosensory canal fused to posttemporal at point of contact with supracleithrum. Scapula broadest dorsally at contact with cleithrum, angling anteriorly to contact coracoid. Coracoid with dorsallyexpanded process contacting cleithrum near point of dorsal flexion and long, thin anterior process contacting ventral margin of cleithrum. Four irregularly-shaped proximal radials. Propterygium with broad cartilaginous base and long, slender process. Fifteen to 16 pectoral-fin rays. First ray about 1/3 length of other rays and surrounded for most of length by propterygium.
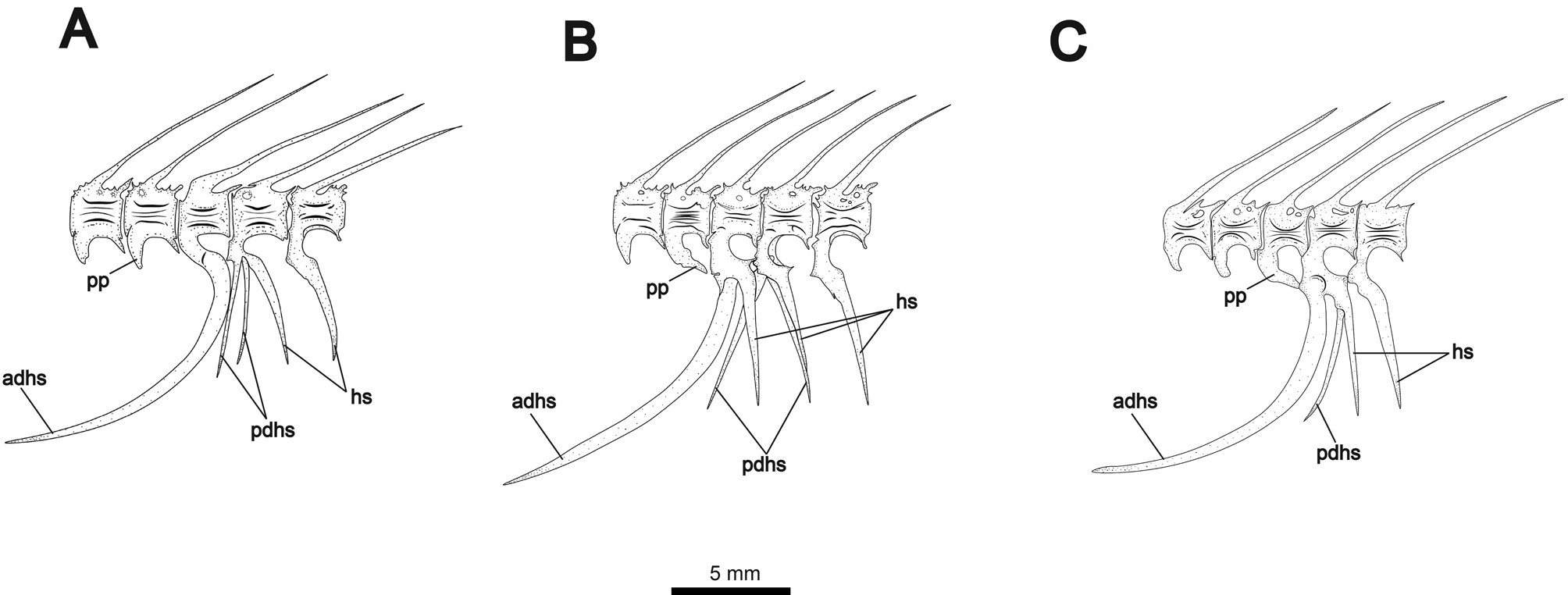
First four vertebrae forming Weberian apparatus. Fifth vertebra articulating with first rib. First rib robust and greatly expanded dorsoanteriorly, contacting enlarged fourth parapophysis forming ventrally-angled concavity. Fourteen to 15 precaudal vertebrae, counting the last vertebra not articulating with the large anterior-displaced hemal spine (demarcating the posterior margin of abdominal cavity). Alternate configurations of displaced hemal spines shown in Fig. 11 View FIGURE 11 . Anterior displaced hemal spine long and saber-shaped, curving anteroventrally. Anterior displaced hemal spine may be the only ventrally-extending element of its associated vertebra ( Fig. 10A View FIGURE 10 ), or it may articulate anterior to a single normal hemal spine ( Fig. 11B View FIGURE 11 ). Additionally, the anterior displaced hemal spine may articulate with a vertebra bearing both a normal hemal spine and a posterior displaced hemal spine ( Fig. 11C View FIGURE 11 ). The parapophysis of the preceding (anterior) vertebra may extend posteroventrally to contact anterior displaced hemal spine dorsally. One or two posterior displaced hemal spines present, straight and usually angled anteriorly. One specimen (shown in Fig. 11B View FIGURE 11 ) shows a single anteriorly-angled posterior displaced hemal spine and two closelyjoined spines in the posteriorly angled position of the rest of the caudal hemal spines. Fig. 11A View FIGURE 11 also shows three hemal spines articulating to one vertebra, but with two displaced spines angled anteriorly.
Anal fin with 167–181 rays (mode = 178). Anal fin pterygiophores longer than displaced hemal spines. Pterygiophores broadened ventrally into thin, symmetrical vanes above articulating head. Caudal fin small and lanceolate in undamaged or fully regenerated specimens, with 12–16 (mode = 14) rays. Number of rays higher in specimens with regenerated caudal fins.
No secondary sexual dimorphism was observed in the cranial morphology or dentition of this species. Females could be recognized externally by their swollen abdomens or internally by mature ovaries containing large (1–2 mm in diameter), orange-yellow translucent eggs. Adult males were recognized by their opaque, cream-colored testes.
Color pattern, Dense brown chromatophores covering dorsum and sides, becoming more diffuse on skin overlaying pterygiophore musculature. Chromatophores arranged in series between pterygiophores forming thin, narrowly-spaced dark bars. Margin of upper lip and lower jaw white or cream-colored with few chromatophores. Opercle darkened by underlying gill filaments. Ground color in life gray to brown dorsally and pale pink over analfin pterygiophores. Head, dorsum, and dorsal organ speckled with pale-colored tuberous electroreceptor pores. First five lateral-line pores prominently visible against dark brown scaleless region behind head. Subdermal supratemporal lateral line canal visible externally as short, pale, irregularly-shaped lines. Pectoral fin hyaline at base, becoming dark over most of length. Distal margin of anal fin dark brown to black. Caudal fin uniformly dark, darker than caudal peduncle. Color of alcohol-preserved specimens similar to in life, but with pink and white hues replaced by pale yellow or cream.
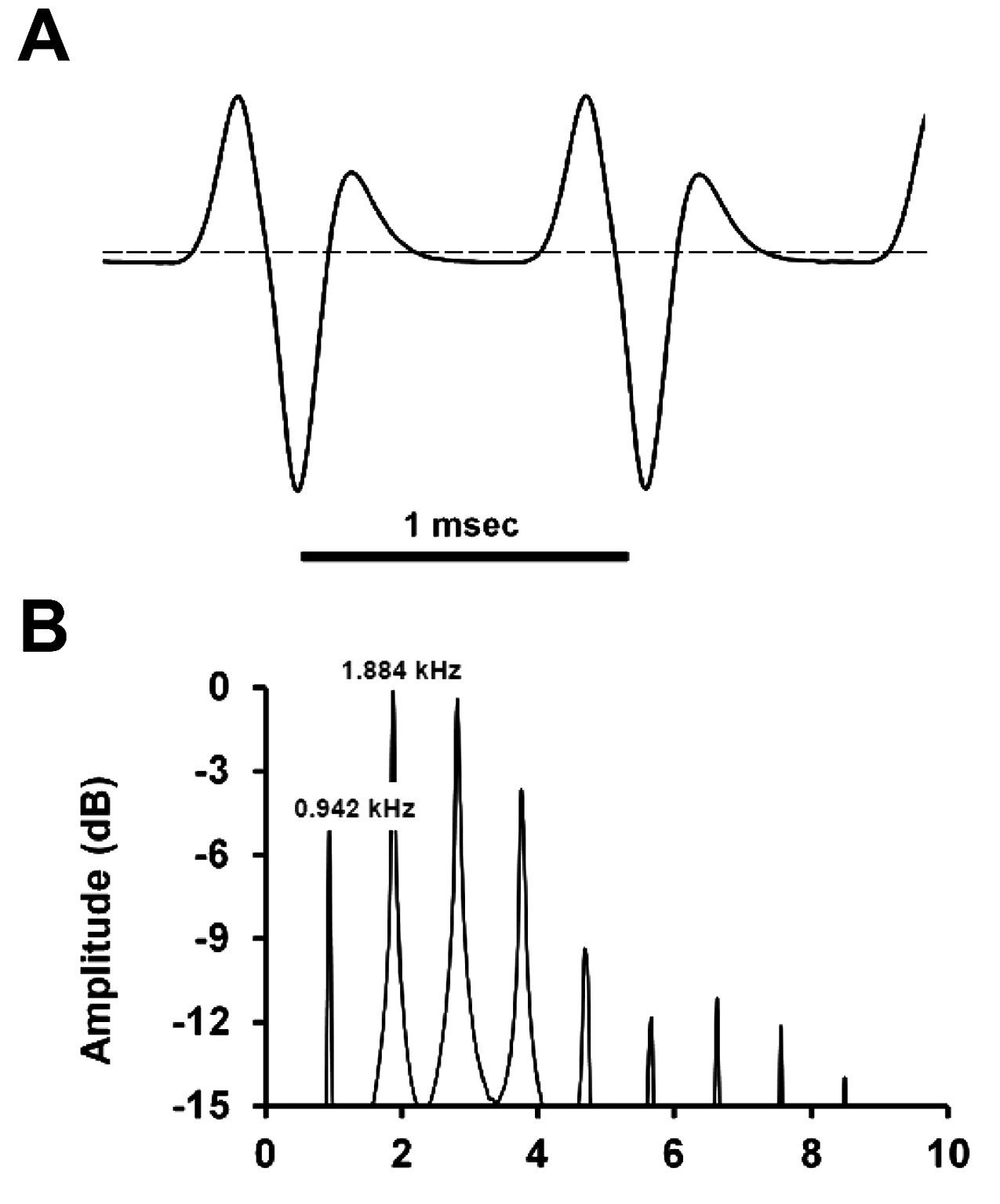
Electric organ discharge. The ht-EOD comprises a wave-type waveform ( Fig. 12A View FIGURE 12 ) with two high-energy positive components and one low-energy negative component in each cycle, with five crossings of 0 V. The mid portion of the cycle contains a brief period (» 0.5 V) during which voltage is approximately constant and only slightly below 0 V. The fundamental frequency is 0.942 kHz. The power spectral density computed exhibits a harmonic content ( Fig. 12B View FIGURE 12 ), typical of wave-type ht-EODs, with the peak power frequency shifted from the dominant frequency to the first harmonic at 1.994 kHz (twice the dominant frequency). The shifting of peak power to the first harmonic is caused by the short-period dominant positive component in each ht-EOD cycle.
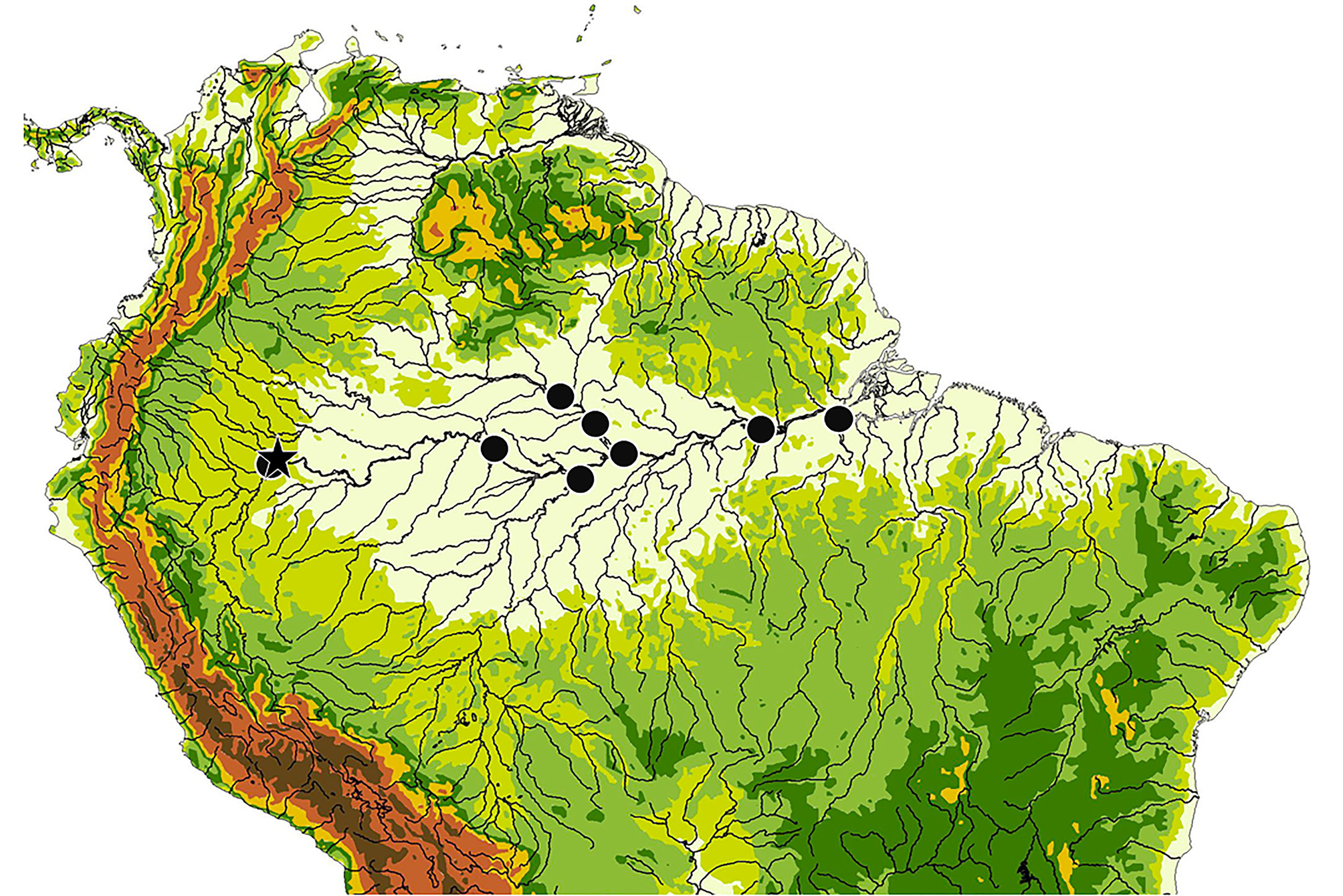
Ecology. Of the 59 specimens of Melanosternarchus amaru examined in this description, the majority (52) were collected from blackwater rivers. Six were collected from whitewater, and one from a clearwater river. This distribution suggests that this species exhibits a habitat preference for black or low-conductivity waters. The three specimens from the main channel of the Amazon were collected very near the mouth of the Rio Tapajós, a site with substantial clearwater input. Two specimens were taken from the lower Rio Purus, but the lower reaches of this river are known to have lower conductivity and suspended matter than other large, whitewater tributaries or the main channel of the Amazon ( Gibbs, 1967; Ríos-Villamizar et al., 2016). One specimen was taken from the lower Rio Japurá. The Rio Japurá is a hybrid whitewater-blackwater river below its confluence with the Paraná Aranapú (a major channel which transports water from the Rio Amazonas to the Japurá). Extensive benthic sampling of the whitewater Amazon River near Iquitos and Tefé during both high and low water has not produced any specimens of M. amaru .
Little is known about the feeding habits of Melanosternarchus . Gut-content analysis of six specimens revealed a majority (4/6) to contain unidentified insect larvae. Beetle larvae (Coleoptera) and caddisfly larvae (Trichoptera) were each present in half of the stomachs, while trichopteran pupae were found in two stomachs. Larval mayflies (Ephemeroptera), stoneflies (Plecoptera), and chironomid midges were each found in only a single specimen. Additionally, the stomach of the largest specimen of M. amaru examined in this description contained scales and fin rays from the severed and swallowed caudal fin of another apteronotid electric fish.
Distribution. The collection localities for this species are summarized in Fig. 13 View FIGURE 13 . The majority of samples of this species were collected from the Rio Nanay near Iquitos (see Fig. 14 View FIGURE14 ) in the Maynas district of Loreto, Peru, but its range is extensive throughout the Amazon basin. Within Amazonas, Brazil, a single specimen was collected from the mouth of the Rio Japurá near Tefé. One lot (n=2) was collected from the Rio Purus, about 40 km upstream from its mouth. Several specimens were collected from the Rio Negro from as far as 50 km above the mouth of the Rio Branco, from the vicinity of São Francisco de Assis, and from the river’s confluence with the Amazon at Manaus. Within Pará, Brazil, two lots were collected from the Amazon River near the mouth of the Tapajós near Santarém and one specimen was captured from near the mouth of the Rio Xingu at Porto de Moz.
Etymology. The species name is from the Quechuan amaru , a mythical serpent, referring to the snakelike shape of this fish. A noun in apposition.
Phylogenetic relationships. Our maximum likelihood phylogram ( Fig. 15 View FIGURE15 ) places Melanosternarchus as sister to Compsaraia , and also places the clade Compsaraia + Melanosternarchus as sister to Pariosternarchus Albert & Crampton. This topology indicates that Sternarchogiton porcinum Eigenmann & Allen is the sister to these three genera, but this relationship has a low bootstrap value of 41. This tree also shows Sternarchella Eigenmann to be sister to Sternarchogiton nattereri Steindachner and S. labiatus de Santana & Crampton , with a bootstrap value of 40. Sternarchogiton preto de Santana & Crampton is well-supported as the sister to all other Navajini.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |