Litoria chrisdahli, Richards, Stephen J., 2007
|
publication ID |
https://doi.org/ 10.5281/zenodo.177512 |
|
DOI |
https://doi.org/10.5281/zenodo.5662663 |
|
persistent identifier |
https://treatment.plazi.org/id/B003F444-515D-0B08-FF2D-F8EEFE18D2CF |
|
treatment provided by |
Plazi |
|
scientific name |
Litoria chrisdahli |
| status |
sp. nov. |
Litoria chrisdahli sp. nov.
( Figs 1–4 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 )
Type material. Holotype. SAMA R62510 (FN SJR8886). Adult ɗ, Wamangu, East Sepik Province, Papua New Guinea (03o 47.224S, 143o 39.125 E, ~ 180 m a.s.l), collected by Chris Dahl on 28.10.2004.
Paratypes. UPNG 10047 (FN SJR8866), UPNG 10048 (FN SJR8937), SAMA R62506 (FN SJR8679), R62507 (FN 8883), R62509 (FN 8885) Adult ɗɗ, SAMA R62508 (FN SJR8884) Adult Ψ, same data as for holotype,
Diagnosis. Distinguished from all other Australopapuan hylid frogs by a combination of the following features: 1) distinct dermal spine on the snout in males and females, 2) moderately large size (males 37–38.5 mm, a female 45.6 mm), 3) well-developed dermal crenulations along outside of limbs and prominent dermal folds above vent, 4) fully-webbed fingers and 5) advertisement calls produced in groups of 2–6 distinctly pulsed notes in which pulses are clumped and pulse rate and structure are remarkably variable.
Description of holotype. Adult male with vocal slits, calling when collected. Measurements and proportions are presented in Table 1 View TABLE 1 . Head flattened, width slightly less than one-third of SVL (HW/SVL 0.312); snout protruding, with prominent (1.2 mm) anteriorly directed dermal spike. Loreal region oblique, slightly concave, canthus rostralis strongly curved; nostrils lateral and concealed from above by prominent snout cants; internarial span much greater than distance between eye and naris (EN/IN 0.76), eye large, its horizontal diameter slightly greater than eye-naris distance; pupil horizontal. Tympanum small (EAR/EYE 0.49) but prominent, its superior margin hidden by prominent, strongly curved supratympanic fold. Tongue narrow with shallow posterior notch. Vomerine teeth in two small groups between choanae; a pair of long vocal slits from near angle of jaw extend half way to anterior of mouth. Fingers long, flattened, extensively webbed with large terminal discs. Webbing extends to base of disc on fingers 2 and 4, reaches disc on finger three by a broad fleshy fringe, and to distal edge of subarticular tubercle on finger one. Subarticular tubercles prominent, distal tubercles on fingers distinctly bi-lobed. A conspicuous crenulated fringe along outer edge of fourth finger extends along forearm to slightly before elbow where it is reduced to series of low, pale tubercles. Relative lengths of fingers 3>4>2>1; a low, finely granular nuptial pad on dorsal surface of thumb. Hind limbs rather short (TL/SVL 0.495), toes in decreasing order of length 4>3=5>2>1, webbing reaches discs of all toes except fourth where it extends to tubercle at base of penultimate phalanx and extends to disc as broad fringe. Inner metatarsal tubercle well developed, no outer metatarsal tubercle. A conspicuous crenulated dermal fringe from disc of fifth toe to heel, most strongly pronounced along tarsus, continues as a short row of tubercles approximately 5 mm along edge of tibia. Two large, semi-circular dermal flaps below vent. Dorsum shagreened, with small irregular ridges and tubercles. Ventral surface of hind limbs and fore limbs smooth; throat, chest, abdomen and posterior half of femora granular; small triangular dermal lappets on lateral margin of lower jaw.
In preservative dusty grey dorsally with small flecks of darker grey; tiny flecks of ivory-white on outside of limbs and around vent. Head with slightly darker grey markings than rest of dorsum. Dorsally fingers 1–2 and toes 1–3 white, finger 3 grey with white circum-marginal edge, finger 4 and toes 4–5 predominantly grey; all toes with ivory fringes. Throat white with sparse brown pigmentation on lateral edges of lower jaw; venter becoming cream posteriorly. Hidden surfaces of thighs dark brown, with narrow white stripe along hidden lateral surface of tibiae. Palmar and plantar surfaces with dense stippling of dark grey-brown pigmentation; dermal fringes and flaps ivory-white.
Colour in life. Based on photographs in life of paratype SAMA R62506, dorsum and dorsal surfaces of limbs flesh-toned with numerous lime-green blotches and several large, brown blotches dorsally and dorsolaterally. Brown pigment most prominent on dorsal surface of head, knee, and laterally on body where it forms a broad, strongly scalloped band between axilla and vent. Green pigmentation concentrated on edge of upper eyelid and on forearm. Dorsal and ventral portions of iris flesh-coloured with brown flecks and reticulations, dark chocolate-brown pigmentation concentrated around pupil, particularly anterior and posterior of pupil. Finger and toe webbing flesh-coloured, mottled with brown and with small green flecks; crenulated folds along limbs flesh-coloured. Tympanum flesh-coloured and mottled with darker brown and specks of green. Tympanic membrane noticeably darker than pale flesh-coloured skin immediately posterior to it ( Fig. 4 View FIGURE 4 ).
Variation. Measurements of the type series are presented in Table 1 View TABLE 1 . Body size and proportions are similar and all specimens including the female exhibit the anteriorly directed rostral spine. The female is the largest specimen but in all other respects closely resembles the males. All specimens exhibit conspicuous dermal ornamentation on the limbs and jaw, and a pair of large dermal flaps below the vent. There is always a narrow white fold along the hidden surface of the tibiae, and the inner surfaces of the thighs are always dark brown. There may be a dark grey band between the eyes and along the canthus, and another narrow band from the rear of the eye to just behind the angle of the jaws. Ground colour is pale to dark grey, and the extent and pattern of darker pigmentation on the dorsum, and of blue and blue-grey (green in life) flecking and blotches is highly variable, the latter ranging from absent in the holotype to quite extensive on the dorsum and fingers in several paratypes.
FIGURE 5. Call sequence of Litoria chrisdahli sp. nov. holotype (SAMA R62510) recorded at an air temperature of 26o C.
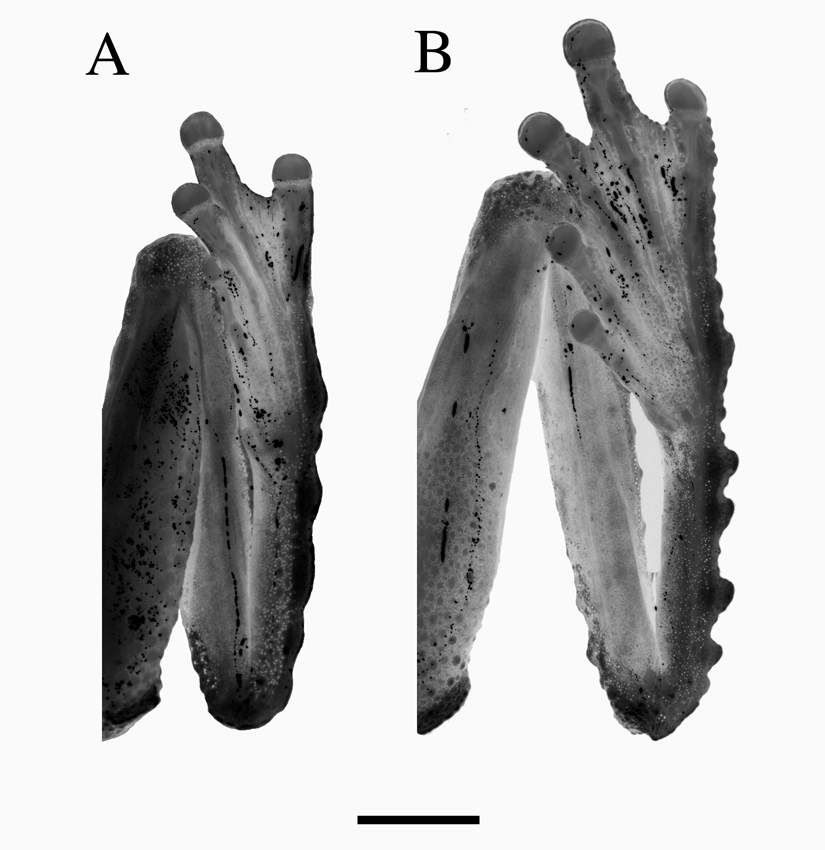
Comparison with other species. Five other species of New Guinean frogs exhibit a prominent rostral spike. Litoria chrisdahli sp. nov. differs from three of these; L. havina , L. mucro and L. pronimia in its much larger size (male SVL 37–39 mm vs <35 mm), the possession of well-developed dermal lappets on the limbs, and conspicuous flaps of skin below the vent. Females further differ from these species in possessing a rostral spike (absent in females of havina , mucro and pronimia ). Litoria chrisdahli sp. nov. most closely resembles, and is clearly most closely related to, L. prora and L. humboldtorum from which it differs in its smaller size (males 37–38.5 vs> 40 mm SVL) and in having a different advertisement call (see below). It further differs from both of these species by its relatively reduced dermal ornamentation. The tarsal ornamentation of L. chrisdahli sp. nov. and L. prora is compared in Fig. 3 View FIGURE 3 . The only known female specimen of Litoria hilli Hiaso & Richards 2006 has a small dermal ‘flap’ on the snout that is much shorter than the spike exhibited by males and the female specimen of L. chrisdahli .
Advertisement call. The advertisement call of Litoria chrisdahli sp. nov. is a series of 2–6 rather harsh, distinctly pulsed notes. Calls are remarkable for exhibiting extreme variability in patterns of pulse structure within notes. Like L. humboldtorum and L. prora the new species produces both ‘long notes’ and ‘short notes’ (see Günther 2006), and ‘long notes’ have a distinct sub-note structure in which pulses are distinctly clumped (Fig 5.). However unlike these species the pattern of pulse production by L. chrisdahli sp. nov. in long notes is so variable that ‘sub note’ structure within a call sequence is different between nearly every note within a call (e.g. Figs 5, 6). Within notes pulses may be produced singly, in couplets, triplets, or in ‘clumps’ of up to 10 pulses ( Fig. 6 View FIGURE 6 ) and they may be produced at regular or variable intervals so that pulse rate within and among notes varies widely. This exceptionally variable pulse structure produces a distinctive and rather harsh spluttering effect that is not evident in L. humboldtorum or L. prora ( Günther 2006) . A series of 15 calls produced by the holotype (SAMA R62510) at an air temperature of 26o C, consists of 2–6 distinctly pulsed notes produced at intervals of 4.40– 19.80 s (mean = 12.87, SD = 4.64, N = 14). Call length is extremely variable, depending on the number of notes produced and it is often difficult to determine where one call finishes and another starts. Ten full call sequences made by the holotype lasted for 1.20– 4.10 s (mean = 2.93, SD = 0.99) and notes were produced at a rate of 0.9–1.8/s (mean = 1.30, SD = 0.28, N = 10). From the five best calls 17 long notes lasted 0.35– 0.70 s (mean = 0.48, SD = 0.10) and two short notes lasted 0.04 s. Pulse rate was extremely variable within and among these notes. Pulse rate in the long notes was 63.95–178.24/s (mean = 100.51, SD = 30.79, N = 17) and was 252 and 309 pulses/s in two short notes. Dominant frequency of the 19 notes analysed above was 2040–2546 Hz (mean = 2282, SD = 135.4). A call sequence is illustrated in Fig. 5 and the variation in pulse structure is illustrated in more detail in Fig. 6 View FIGURE 6 . The advertisement call of Litoria chrisdahli sp. nov. is strikingly different from that of L. prora and L. humboldtorum ; calls of the latter two species were analysed by Günther (2006). L. humboldtorum normally utters one long note followed by two shorter notes, with long intervals between calls, a pattern not observed in the new species. It also has a much lower dominant frequency than L. chrisdahli (1.8 vs ~ 2.2 kHz). Calls of L. prora consist of 2–10 notes with highly variable intervals between calls, and with a general pattern of shorter notes followed by longer notes ( Günther 2006). Calls of L. prora also lack the extreme variability in pulse structure exhibited by L. chrisdahli sp. nov., and have a distinctly melodious call that is quite unlike the harsh, and slightly ‘chattering’ or ‘spluttering’ sound produced by this species.
Natural history. The type locality is adjacent to the Nakam River. The habitat is a complex patchwork of secondary lowland rainforest, patches of bamboo, coconut plantations, cleared forest and gardens, and sago swamps. A number of large, buttressed trees remain. The forest is prone to inundation after several days of rain. Male Litoria chrisdahli sp. nov. called predominantly on relatively cool, wet nights. They were common around pools and swamps (particularly sago swamps) in severely disturbed habitats. Several males were found calling from sago palm leaves about 3–5 m high in the evening after rain; others were found in lowland rainforest near the sago swamps, calling from between 50 cm – 2 m high on leaves. The female contains large, unpigmented pale yellow eggs suggesting that the reproductive behaviour of this species, like L. prora , involves the deposition of eggs on leaves above water ( Richards 2002).
Etymology. The new species is named for Mr Chris Dahl, who collected the type series, in recognition of his dedication and hard work that resulted in the documentation of this species and many other interesting frogs in northern New Guinea.
| SAMA |
South Australia Museum |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.