Liolaemus burmeisteri, Avila, Luciano Javier, Perez, Cristian Hernan Fulvio, Medina, Cintia Debora, Sites, Jack Walter & Morando, Mariana, 2012
|
publication ID |
https://doi.org/ 10.5281/zenodo.213183 |
|
DOI |
https://doi.org/10.5281/zenodo.5674762 |
|
persistent identifier |
https://treatment.plazi.org/id/14648266-E35E-FFB6-28C3-F92BFD74E8FD |
|
treatment provided by |
Plazi |
|
scientific name |
Liolaemus burmeisteri |
| status |
sp. nov. |
Liolaemus burmeisteri sp. nov.
Holotype. — MLP.S 2612 ( Figs. 1 View FIGURE 1 , 2 View FIGURE 2 ), an adult male from Ruta Provincial 41, 7 km S Caepe Malal, Chos Malal Department, Neuquén, Argentina (37º13’51.4”S, 70º22’24.3”W, 1037 m), collected 19 January 2007.
Paratypes. — MLP.S 2613, LJAMM 5241–5242 (male) and MLP.S 2614-2617 (female) from same locality as holotype. Collected 20 January 2003. LJAMM 7639, 7641, 7646 (male) and 7637–7638, 7640, 7642–7645, 7647 (female) from same locality as holotype, collected 19 January 2007.
Liolaemus bur- Liolaemus Liolaemus Liolaemus Liolaemus Liolaemus Liolaemus shi- Character meisteri antumalguen chillanensis elongatus smaug choique tan
sp.nov. (n = (n = 11) (n = 99) (n = 21) (n = 22)
19)
Max. SVL 85.2 107.7 70.3 94.7 68.3 90.7 94.7 (mm)
SAM 76.2±2.7(70- 77.0±3.6(68- 81-95 77.5±4.0(68- 74.1±2.6(70- 74-88 78.1±3.9(71-
81) 82) 87) 79) 85) Diagnosis. — Liolaemus burmeisteri can be easily distinguished from all other members of the Liolaemus elongatus clade, by its light brown/ochre general coloration not found in any of the other species and by its homogeneous dorsal pattern without bands, stripes or spotted areas ( Table 1). Liolaemus burmeisteri lacks of a well-defined bodybanding pattern and dark ochre/green, or black general coloration, typical of L. elongatus . Liolaemus smaug has darker coloration and fewer numbers of dorsal scales without overlapping (64–75, 0 = 70.3 vs 76–85, 0 = 81.1 in L. burmeisteri ) and higher numbers of ventral scales (107–119, 0 = 111.9 vs 99–110, 0 = 104.7 in L. burmeisteri ) with little overlap. Liolaemus smaug has a dorsal banding pattern characterized by a very variable but always present vertebral band ranging from a well marked gray-ochre zone to a dark spotted area, and a very wide and dark lateral band, all traits absent in live specimens of L. burmeisteri . Liolaemus chillanensis has a different dorsal coloration pattern, with a vertebral band formed by dark spots, more ochre dark general coloration, more darker lateral bands, and more scales around midbody (81-95 vs 70-81). Liolaemus antumalguen is a larger species (maximum SVL 107.7, 0 = 94.4 vs 85.2 mm, 0 = 74.5), with more enlarged neck pouches, and with fewer scales along
the dorsum and little overlap with L. burmeisteri (70-78, 0 = 73.0 vs 76-85, 0 = 81.1). General coloration is darker in L. antumalguen , usually with a dorsal pattern of black dots, and black head and belly, and tail without any pattern of tail rings, characteristics never found in L. burmeisteri . Liolaemus shitan has fewer numbers of dorsal scales (66–78, 0 = 70.6 vs 76–85, 0 = 81.1 in L. burmeisteri ) and higher numbers of ventral scales (106–129, 0 = 120.6 vs 99–110, 0 = 104.7 in L. burmeisteri ) with little overlap. Liolaemus shitan has a general melanic coloration without any apparent pattern, a coloration trait absent in L. burmeisteri . Liolaemus choique has higher numbers of ventral scales (118–135 vs 99–110 in L. burmeisteri ) without overlapping. General coloration is yellow in Liolaemus choique with a dorsal pattern of vertebral and lateral black melanic bands never found in L. burmeisteri .
Description of holotype. — Adult male. SVL 85.2 mm, total length 210.2 mm; tail incomplete, regenerated (125 mm length). Axilla–groin distance 35.2 mm. Head 19.2 mm long (from anterior border of auditory meatus to tip of snout), 16.6 mm wide (at anterior border of auditory meatus), 9.9 mm high. Arm length 23.1 mm. Tibia length 17.7 mm. Foot length 23.2 mm (ankle to tip of claw on fourth toe). Dorsal head scales irregular, some smooth and others with small holes (not scale organs), irregularities appearing more frequently in parietal–temporal areas. Scale organs more abundant in the anterior head region than in the parietal and temporal region. Nineteen scales between rostral and occiput (at level of anterior border of auditory meatus). Rostral scale wider (4.0 mm) than high (1.3 mm). Two postrostrals. Four internasals, irregular. Five frontonasals, irregular. Four prefrontals, irregular. Frontal scale divided longitudinally, forming two scale rows between circumorbitals. Four scales between frontal and rostral. Interparietal pentagonal, similar size to parietals. Interparietal surrounded by seven scales, with a central, small, and obscure ‘‘parietal eye’’ in the scale center. Supraorbital semicircles complete. Seven enlarged supraoculars. Five scales between frontal and supercilliaries. Seven supercilliaries, irregular flattened and elongated. Nineteen temporals on each side, smooth and protruding with 1–3 scales organs. Canthal scales separated from nasal by one scale. Loreal region concave. Seven scales surrounding nasals on each side. Nasal in slight contact with rostral on each side. Orbit with 16 upper and 14 lower ciliaries. Orbit diameter 2.5 x 4.2 mm (measured between upper and lower ciliaries). Subocular scale elongate. Preocular fragmented on two scales. Six lorilabials, three in contact with subocular. Six supralabials on each side. Fourth supralabial curved upward posteriorly but fourth not in contact with subocular. Infralabials, four, first scale twice as high as posterior infralabials. Postrostrals, internasals, frontonasals, prefrontals, loreal, lorilabials, supra– and infralabials with conspicuous scale organs. Three outwardly projecting scales along anterior border of auditory meatus. Auditory meatus about twice as high (3.9 mm) as wide (1.7 mm). Lateral scales of neck granular with skin below appearing slightly inflated. Antehumeral, longitudinal, and postauricular neck folds distinct, oblique less conspicuous, gular incomplete, rictal not present. Thirty–seven scales between auditory meatus and antehumeral fold (counted along longitudinal fold). Scales of dorsal neck region similar to dorsals. Mental wider (3.9 mm) than long (2.1 mm), followed posteriorly by two rows of chinshields with four scales in the left side and six on right side. Chinshield rows four scales in contact with mental. Throat scales between chinshields slightly juxtaposed, strongly imbricated toward auditory meatus. Fourty–nine gulars between auditory meatus. Eighty–two dorsal scales between occiput and anterior surface of thighs. Thirty–six longitudinal keeled scales rows. Scales become larger and less keeled through flanks. Flank scales with one scale organ at the tip. Scales small and granular around limb insertions. Eighty scales around midbody. Ventral scales of similar size to dorsals, but smooth and round, 104 between mental and cloacal aperture. Precloacal scales slightly larger than ventrals. Five precloacal pores.
Tail quadrangular in cross section near cloacal area, becoming oval to round in the rest. Caudal scales in discernible annuli. Dorsal and upper lateral caudals scales keeled, imbricate, mucronate, and outward projecting. Lower lateral scales moderately keeled and mucronated, and ventral scales smooth. Suprabrachials, imbricate, moderately keeled; prebrachials imbricate, weakly keeled, grading into smaller subimbricate or granular infrabrachials. Supra–antebrachials imbricate, very weakly keeled; post–antebrachials imbricate, moderately keeled with 1–3 mucronated keeled toward the ventral region; pre–antebrachials imbricate, smooth; and infra–antebrachials imbricate, becoming of smooth in the posterior region to moderately keeled and mucronate at the anterior region. Suprametacarpals imbricate, smooth; inframetacarpals imbricate, keeled, 2–3 mucronate. Supradigitals of manus smooth, wider than long; subdigitals with three keels, each terminating in a short blunt mucron, numbering: I: 10, II: 15; III: 22; IV: 22; V: 16. Claws robust, moderately curved, opaque brown. Suprafemorals imbricate, moderately keeled; prefemorals and infrafemorals imbricate, smooth. Postfemorals small, granular. Supra– and pretibials imbricate, moderately blunty keeled; infratibials imbricate, smooth. Supratarsals imbricate moderately keeled; and infratarsals imbricate, 3– keeled, mucronate. Supradigitals of foot smooth, wider than long; Subdigital scales keeled, becoming of 4–keeled in the posterior region of digit to 3–keeled in the tip, mucronate, numbering: I: 13; II: 18; III: 25; IV: 30; V: 19. Claws robust, moderately curved, opaque brown.
Color of holotype in life. — General body coloration uniform, light brown or light ochre. Dorsal and lateral head scales, with scattered few dark brown smudges on postrostral, internasal, frontonasal, prefrontal, temporals and frontal scales. A thin black line on the superior border of the subocular scale. Infralabial scales whitish with small dark brown areas. Dorsal scales between occiput and cloacal region light brown/ochre without definite pattern, speckled with very small (one scale) white or black spots. Tail light brown, weakly ringed with ochre bands. Body lateral region with a dark brown band between axilla and groin, darker than dorsal scales, speckled with a few white spots (one scale). Upper limb surfaces ochre with reticulated of dark brown. Ventral scales of throat, neck, chest, belly and forelimbs light gray with some melanic sectors, more marked in belly midline. Ventral scales of lower belly and femoral region, bright yellow. Ventral scales of cloacal region and tail whitish.
Color of holotype in preservative. — After five years in preservative, the dorsal coloration of the head, dorsum, body flanks and tail become darker while maintaining the contrast, but the white spots largely disappeared. Ventral scales of throat, neck, chest, belly and forelimbs darkened, and distinctive yellow ventral coloration of the femoral region and belly turned dark gray. General pattern of coloration become more marked and general coloration disappeared.
Variation. — Liolaemus burmeisteri adults ranging from 60.5–85.2 mm SVL. As in other members of this group, no obvious body size sexual dimorphism was observed (except tail expansion in cloacal areas of males and slightly smaller head width in females). In seven males ( Table 2 View TABLE 2 , Fig. 3 View FIGURE 3 ): SVL: 77.8–85.2 mm. Head length: 17.3– 19.25 mm. Head width: 13.6–16.6 mm. head height: 8.15–9.95 mm. Foot length: 23.2–24.9 mm. Tibial length: 17.4–18.9 mm. Arm length: 21.9–25.4 mm. Midbody scales: 73–81. Dorsal scales (between occiput at the anterior margin of auditory meatus and anterior surface of thighs): 76–83. Ventral scales 101–109. Fourth toe lamellae: 29– 31. Supralabial scales: 6–8. Infralabial scales: 5. Cloacal pores: 0–5. In twelve females ( Table 2 View TABLE 2 , Fig. 4 View FIGURE 4 ): SVL: 58.3–78.9 mm. Head length: 13.6–16.7 mm. Head width: 10.9–13.8 mm. head height: 6.30–8.15 mm. Foot length: 20.5–23.0 mm. Tibial length: 13.9–17.6 mm. Arm length: 18.7–23.4 mm. Midbody scales: 70–79. Dorsal scales: 76–85. Ventral scales: 99–110. Fourth toe lamellae: 27–31. Supralabial scales: 7–9. Infralabial scales: 4–5. For nineteen individuals (males and females): dorsal scales: 81.15 ±2,89 (76–85). Interparietal scale usually pentagonal shaped, bordered by 6–8 scales. Supralabial scales 6–9. Infralabial scales 4–5. Third finger lamellae ranges 19–23. Fourth toe lamellae ranges 27–31.
Dorsal and lateral coloration in life is almost identical in all individuals and varies only in intensity. Yellow ventral coloration of males in femoral and lower belly region is variable in extent and intensity in the areas indicated. In preservative, dorsal coloration of all individuals fades to a darker, although all retained the contrast between back and head and flanks and in some individuals scattered dorsal white spots disappeared. All distinctive femoral areas and lower belly coloration fades in preservative from yellow to dark gray.
Etymology. — The specific name is to honor Karl Hermann Konrad Burmeister, a German naturalist, paleontologist and zoologist. Carlos Germán Conrado Burmeister (as he was known in Argentina) was born in Stralsund, Germany in 1807 and past away in Argentina in 1892 after a prolific work with near 300 publications about animals, plants, geology, and paleontology of South America, including its Description Physique de la République Argentine d’après des observations personnelles et étrangères (with a version in German). He was director of the Museo Público de Buenos Aires (now Museo Argentino de Ciencias Naturales Bernardino Rivadavia) for 30 years and was in charge of the organization of the National Academy of Sciences in Córdoba, founded by the Argentinean president Domingo F. Sarmiento.
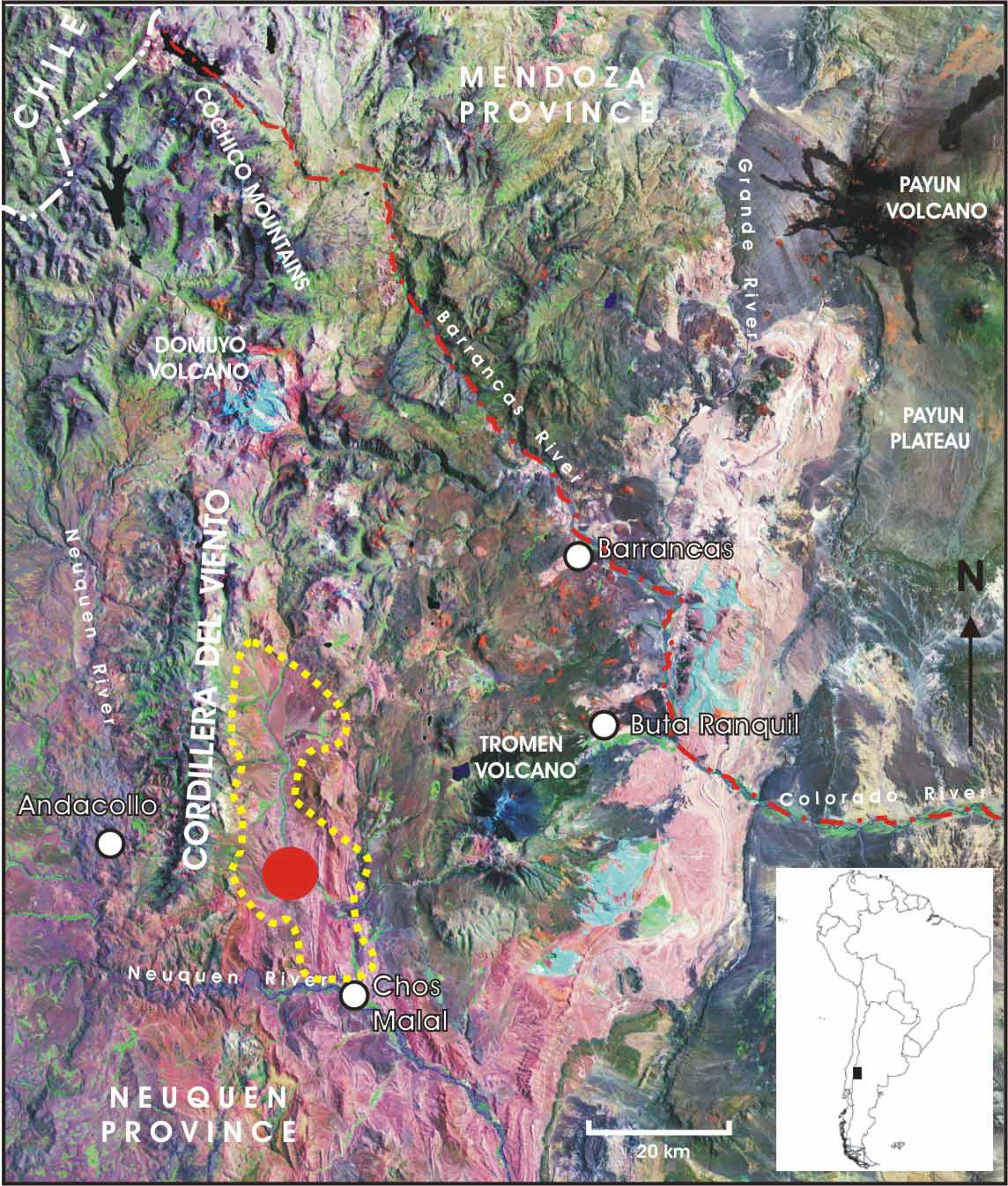
Distribution. — Liolaemus burmeisteri is known only from the type locality ( Fig. 5 View FIGURE 5 ), Ruta Provincial 41, 7 km S Caepe Malal (37º13’51.4”S, 70º22’24.3”W, 1037 m), northwestern Patagonia, Chos Malal Department, Neuquén Province, Argentina.
Natural history. — The collection area was on a small plateau with sedimentary rocks and sparse vegetation. The type locality is a plateau with rocky soils and with medium to big–sized rocks scattered or rocky outcrops, alternating with areas of loose sand ( Fig. 6 View FIGURE 6 ). This area is only sparsely vegetated, with soil erosion resulting from grazing by goats and horses. However, in undisturbed areas, typical vegetation is composed of plants of the genera Stillingia , Colliguaja , Nassauvia , Haploppapus , Fabiana , Schinus and Stipa , which are representatives of the Payunia District, Patagónica Province, Andino–Patagónico Domain ( Roig 1998). Liolaemus burmeisteri appears to be restricted to rocky areas, similar to many other members of this clade ( Cei 1974, 1979, 1986, 1993; Espinoza & Lobo 2003; Espinoza et al. 2000; Hulse 1979). When active, lizards move across the rocky substratum and bask on horizontal surfaces or on top of medium–sized stones. When disturbed, L. burmeisteri usually attempts to escape into the cracks and crevices in the rocks. Based on analyses of stomach contents, L. burmeisteri feeds on a variety of insects and other small arthropods. In two digestive tracts, we found Orthoptera, Coleoptera, Formicidae, Solifugae, Araenae , sand grains and indeterminate parts of arthropods. These contents are very similar to those found by Videla (1983) in L. elongatus from Mendoza Province. Detailed biological information has been published only for a few phylogenetically related lizards (e.g. Ibargüengoytıa & Cussac 1998; Quatrini et al. 2001; Videla 1983), with observations in Cei (1986), Espinoza & Lobo (2003), Espinoza et al. (2000), Hulse (1979), and Schulte et al. (2000). No conclusive evidence of viviparity can be offered, but all related species of the elongatus clade share this reproductive mode ( Cei 1986; Espinoza & Lobo 2003; Espinoza et al. 2000; Hulse 1979; Schulte et al. 2000). At the type locality, L. burmeisteri was found in syntopy with Homonota darwinii , and no other species of lizards were observed.
Remarks —A mtDNA gene tree analysis, including the new described species as well as other related species and candidate species of this and other related clades is depicted in Fig. 7 View FIGURE 7 . This tree is based on the mitochondrial gene fragment cyt-b (805 bp). Liolaemus burmeisteri is recovered as the sister taxon of L. smaug but with very low support. The petrophilus and kriegi clades also include candidate species and are recovered with strong support (0.95 and 0.99 respectively). Recently a typographic error named this clade as buergeri in the remarks section of the description of L. antumalguen ( Avila et al. 2010) . The objective of this tree is to show the position of this new species in relation with these three related clades. Phylogenetic relationships for these clades, including several lines of evidence, are under study by our research group and a detailed analysis will be published elsewhere.
Males (N= 7) Females (N= 12)
Mean SD Range Mean SD Range antumalguen austromendocinus buergeri capillitas ceii chillanensis curis cristiani dicktracy elongatus flavipiceus gununakuna heliodermis kriegi parvus leopardinus petrophilus punmahuida talampaya thermarun tregenzai tulkas umbrifer villaricensis
Cei, 1974 E E E
Cei, 1975 E B K E K E
Cei, 1979 E K K E K E
Ortiz, 1981 E K K E K E
Cei, 1986 E K K E K E
Cei, 1993 E E E E
Espinoza et al., 2000 E E E E E E Schulte et al., 2000 X X X X X X X
Lobo, 2001 E K E E E K X
Morando et al., 2003 P K P K E K P P P Avila et al., 2004 P K P P E P K P P P P P Lobo, 2005 C C C C Díaz Gómez & Lobo, 2006
Torres-Pérez et al., 2009 E E K
Lobo et al., 2010 E X C X X C E E E C X E E X C X E C C Avila et al., 2010 E E E E E
Species composition of the elongatus clade has changed during the last thirty years ( Table 3), but based on available knowledge and ongoing systematic studies of the petrophilus (Feltrin et al. in prep.) and the elongatus (Medina et al. in prep.) clades; we consider the elongatus clade composition to include: L. elongatus Koslowsky 1896 , L. antumalguen Avila et al. 2010 , L. chillanensis ( Torres-Perez et al. 2009) , three recently described species, Liolaemus smaug , L. choique and L. shitan ( Abdala et al. 2010) , and L. burmeisteri . The three described species by Abdala et al. (2010) were considered in previous studies as populations of Liolaemus elongatus (Morando et al. 2003; Morando et al. 2004).
Liolaemus thermarum was described from a type locality named Termas del Azufre, Malargüe, in Mendoza Province, 10 km from the Peteroa volcano ( Videla & Cei 1996); according to the description of the type locality all lizards were collected in a glacial valley named “Baños del Azufre” along a volcanic relief Azufre-Planchon- Peteroa. Later, Espinoza et al. (2000) cited this species for Laguna Niña Encantada , close to Las Leñas, but Cei & Videla (2003) point out that those samples do not correspond to L. thermarum . Morando et al. (2003) suggested that their sample named Liolaemus sp. 5, from an area close to L. thermarum type locality, could correspond to this species. However, adequate comparison to type specimens deposited in the MACN was not possible due to the poor preservation conditions, and the few characters that could be observed showed some discordance (e.g., absence of precloacal pores in L. thermarum whereas two to four pores are present in Liolaemus sp. 5). Later, we analyzed samples from Rio Grande Valley and Baños del Azufre and considered that Liolaemus sp. 5 from Morando et al. (2003) could be assigned to L. thermarum (e.g. Avila et al. 2010). In the same year, Abdala et al. (2010) described our previously discovered Liolaemus sp. 5 as a new species, Liolaemus smaug , with an extended type locality between Las Loicas and Baños del Azufre. Recently, we were able to obtain a small sample of lizards from north of Baños del Azufre ( L. thermarum type locality), in a glacial valley between this locality and Paso Vergara, east to the volcanic system Peteroa-Azufre Planchon, along the Río de los Ciegos. These lizards, from 5 km north of Baños del Azufre, fit the description of L. thermarum , and they differed from our previously studied specimens referred to as Liolaemus sp. 5 (Morando et al. 2003) and from L. thermarum ( Avila et al. 2010) . We consider that the original description of L. thermarum type locality was not precise enough, and this led Avila et al. (2010), to erroneously assume that specimens analyzed from Baños del Azufre corresponded to L. thermarum . The new evidence indicates that the Baños del Azufre population is the most northern distribution of Liolaemus smaug along the Rio Grande Valley, and the true type locality of L. thermarum , based on the original morphological description of this species, could be located in the valley of Rio de los Ciegos.
In a recent review publication, Lobo et al. (2010) proposed as part of the elongatus clade the following species: L. austromendocinus , L. elongatus , L. flavipiceus , L. gununakuna , L. parvus , L. petrophilus , L. punmahuida , L. thermarum , L. tregenzai ; but no well-supported phylogenetic hypothesis has been proposed for all of these taxa. There are several proposed candidate species, unsampled geographic areas, and more taxonomic and character sampling are needed tor resolve these issues.
TABLE 2. Morphometric and meristic variation in Liolaemus burmeisteri type series. Means and standard deviations (SD) of the main morphometric and meristic characters. Measures in mm and scale in numbers. AGD = Axilla – groin distance, HL = Head length, HW = Head width, HH = Head high, FL = Foot length, TL = Tibial length, AL = Arm length, SAM = Scales Around Midbody, DS = Dorsal Scales, VS = Ventral scales, 4 TL = Fourth toe lamellae, SL = Supralabial Scales, IL = Infralabial scales, PC = Cloacal pores.
| SVL | 80.94 | 2.71 | 77.8–85.2 70.75 | 7.33 | 58.3–78.9 |
|---|---|---|---|---|---|
| AGD | 32.66 | 1.75 | 31.2–35.3 30.78 | 4.82 | 21.0–36.9 |
| HL | 18.32 | 0.62 | 17.3–19.25 15.41 | 1.16 | 13.6–16.7 |
| HW | 15.19 | 0.99 | 13.6–16.6 12.81 | 1.03 | 10.9–13.8 |
| HH | 8.92 | 0.65 | 8.15–9.95 7.32 | 0.67 | 6.30–8.15 |
| FL | 24.18 | 0.77 | 23.2–24.9 22.03 | 0.88 | 20.5–23.0 |
| TL | 18.28 | 0.57 | 17.4–18.9 16.04 | 1.07 | 13.9–17.6 |
| AL | 24.05 | 1.15 | 21.9–25.4 21.55 | 1.59 | 18.7–23.4 |
| SAM | 77.71 | 2.69 | 73–81 75.33 | 2.46 | 70–79 |
| DS | 81.28 | 2.98 | 76–83 81.08 | 2.96 | 76–85 |
| VS | 105 | 2.70 | 101–109 104.58 | 2.96 | 99–110 |
| 4TL | 30.14 | 0.69 | 29–31 29.75 | 1.13 | 27–31 |
| SL | 6.85 | 0.69 | 6–8 7.66 | 0.65 | 7–9 |
| IL | – | – | 5 4.91 | 0.28 | 4–5 |
| PC | 0.85 | 1.86 | 0–5 – | – | – |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.