Lepidosperma viscidum, R. Br., R. Br.
|
publication ID |
https://doi.org/ 10.1016/j.phytochem.2021.112800 |
|
DOI |
https://doi.org/10.5281/zenodo.8269733 |
|
persistent identifier |
https://treatment.plazi.org/id/233B3318-FFFE-0265-FFC0-FF7160B7AF04 |
|
treatment provided by |
Felipe |
|
scientific name |
Lepidosperma viscidum |
| status |
|
2.3. F type propolis and its botanical source from L. viscidum View in CoL
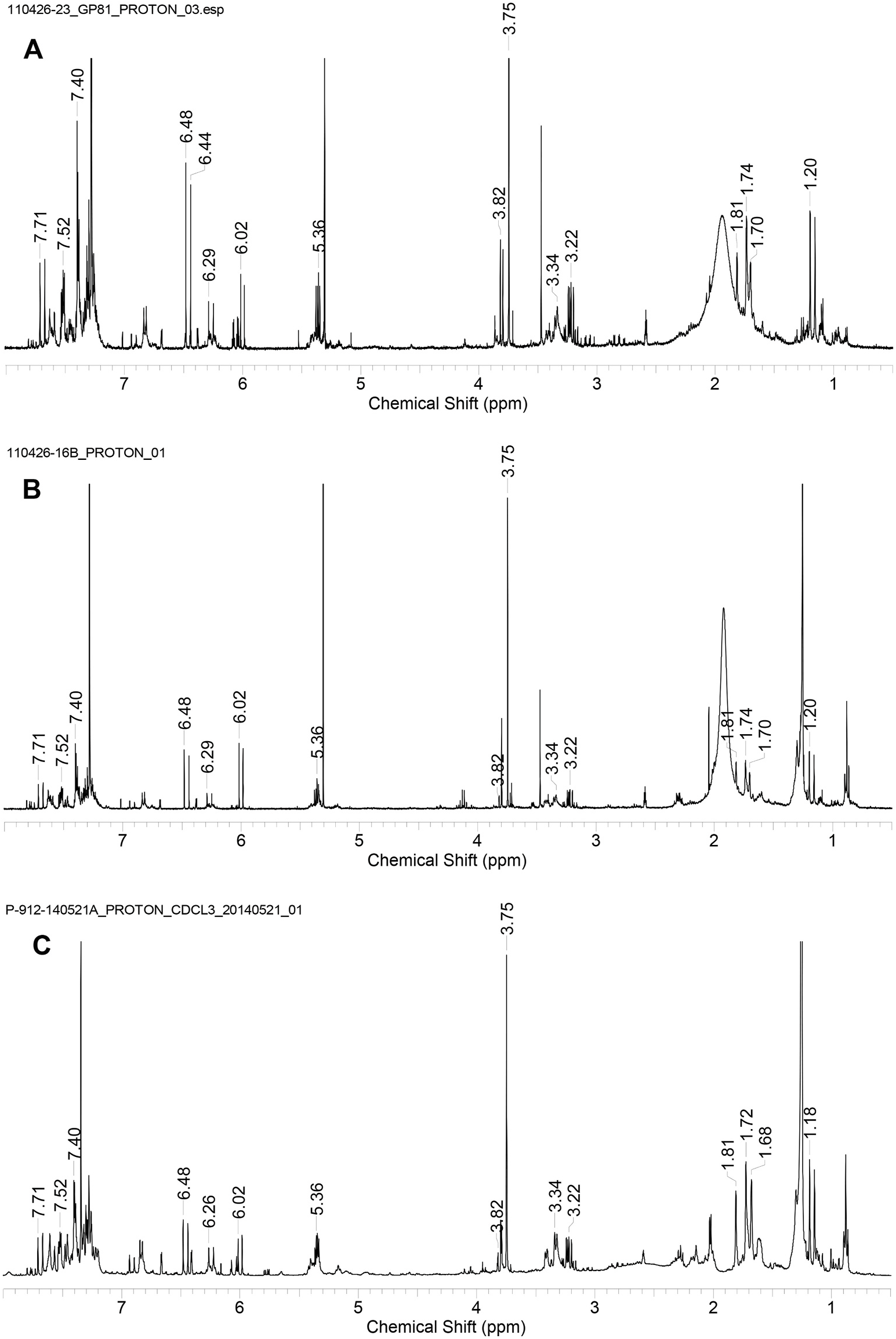
A plant source foraged by honey bees to produce propolis on Kangaroo Island was found to be the resinous exudate of Lepidosperma sp. Montebello ( Duke et al., 2017). The resinous exudates from other species of Lepidosperma genus collected on KI and south-east South Australia were compared by TLC and 1 H NMR analysis with propolis samples. A close match was found for both TLC (Supplementary data, Fig. 3S View Fig ) and 1 H NMR spectra profiles ( Fig. 5 View Fig ) between resin from L. viscidum ( Fig. 4S View Fig ) and propolis samples rich in 1 H NMR signals characteristic of flavanones, designated as F type propolis. F type propolis is relatively uncommon on KI (18 out of 2602 samples) and relatively common in south-east South Australia (4 out of 11 samples). This frequency of appearance is consistent with the uncommon occurrence of L. viscidum on KI and its common occurrence in the areas in South Australia from where F-type propolis samples were collected. L. viscidum resinous leaf and leaf base ethanol extract from Seal Bay, KI, was fractionated by normal-phase short column vacuum chromatography and fractions of sufficient purity were characterised by 1 H and 13 C NMR spectra and mass spectrometry resulting in the identification of five compounds 4, 5, 8, 9 and 10.
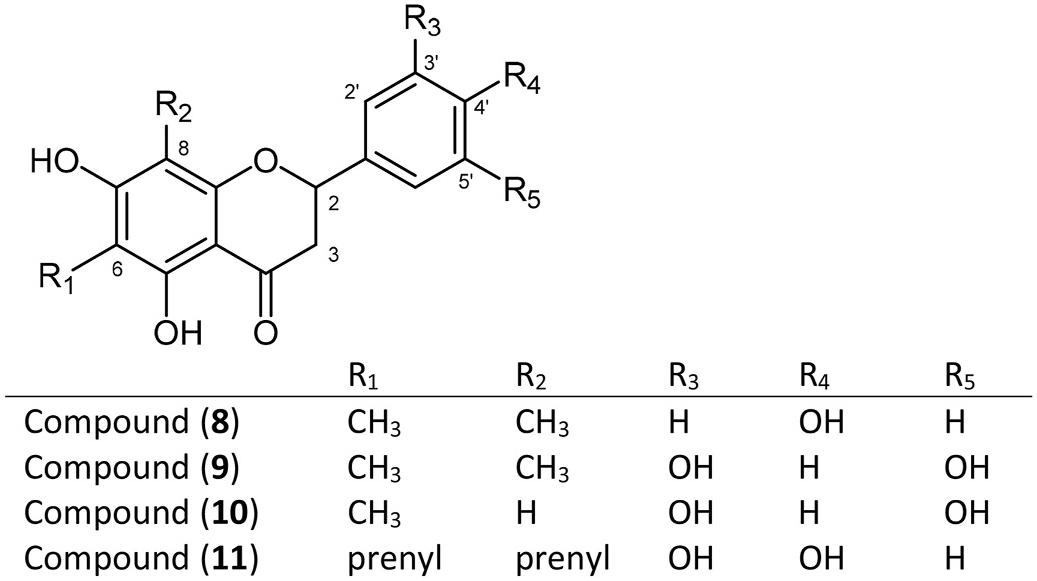
Chemistry of L. viscidum resin is markedly different from that previously observed in another propolis resin sourced from Lepidosperma genus on Kangaroo Island ( Duke et al., 2017; Abu-Mellal et al., 2012). This propolis type has resin sourced from Lepidosperma sp. Montebello, with chemistry extensively investigated ( Duke et al., 2017; Abu-Mellal et al., 2012). The compounds isolated from resins of that species are predominantly C- and O-prenylated hydroxystilbenes or derivatives thereof, many with piceatannol as base structure. By comparison, L. viscidum resin appears to be predominantly 6- or 8-methyl or dimethyl polyhydroxyflavanones 8, 9 and 10 ( Fig. 6 View Fig ); 1 H NMR spectra of less pure fractions also suggests the presence of some O-methylated flavanones. Notably, prenylation appears to be less common in L. viscidum resin compounds, with the exception of the two dihydrochalcones, 4 and 5, isolated.
Compounds similar to 4 and 5 without the 4-hydroxyprenyl substituent, 4,2 ′,4 ′ -trihydroxydihydrochalcone (davidigenin) ( Jensen et al., 1977) and 4,2 ′ -dihydroxy-4 ′ -methoxydihydrochalcone ( Kostrzewa-Susłow and Janeczko, 2012) have 1 H and 13 C NMR spectral results that show good partial concordance with 4 and 5. The most similar structure to 5 reported was 3 ′ -prenyl-4,2 ′ -dihydroxy-4 ′ -methoxydihydrochalcone ( Awouafack et al., 2010). The stereoisomer identified in 4 and 5 was the E configuration. This configuration is reported to be predominant in terminally-hydroxylated prenyl groups in natural products ( Erasto et al., 2004; Nguyen et al., 2012). Compounds 4 and 5 are previously undescribed: a number of 3 ′ -prenyl hydroxydihydrochalcones have been reported from natural sources but no 5 ′ -prenylated structures to date. These 3 ′ -prenyl hydroxydihydrochalcones have been isolated from genera Angelica (Apiaceae) ( Luo et al., 2012a), Artocarpus (Moraceae) ( Jamil et al., 2008), Bacopa (Plantaginaceae) ( Suresh et al., 2010), Broussonetia (Moraceae) ( Luo et al., 2012b), Eriosema (Fabaceae) ( Awouafack et al., 2008, 2010) and Lonchocarpus (Fabaceae) (Borges-Arg´aez et al., 2009), none of these genera being closely related to the Cyperaceae . Limited information on biological activity of these compounds exists, but one is a reported strong free radical scavenger by the DPPH assay ( Jamil et al., 2008) and another has been observed to inhibit aromatase ( Luo et al., 2012b).
The molecular weight of compounds identified as 5,7,4 ′ -trihydroxy- 6,8-dimethylflavanone (farrerol) (8), 5,7,3 ′,5 ′ -tetrahydroxy-6,8-dimethylflavanone (9) and 5,7,3 ′,5 ′ -tetrahydroxy-6-methylflavanone (10) was determined by mass spectrometry. Close matches with literature 13 C NMR and 1 H NMR spectra enabled identification of the structures of these known compounds: farrelol (8) ( Lai et al., 2016); 9 ( Lou et al., 2015); 10 ( Yi et al., 2002; Zhang et al., 2018). Farrerol (8) has a long history in the literature and has been isolated from plants from a widespread number of genera ( Lai et al., 2016). Compound 9 has previously been isolated from Rhododendron dauricum (Ericaceae) ( Wang et al., 2015) as per farrerol, and 10 from the conifer Pseudotsuga sinensis (Pinaceae) ( Yi et al., 2002; Zhang et al., 2018). Neither species belong to families closely related to the Cyperaceae . No reports of biological activity for these compounds were found ( Wang et al., 2015; Yi et al., 2002; Zhang et al., 2018). F type propolis and its resin source L. viscidum are a promising source of a diverse range of flavanones of potentially useful biological activity, and of farrerol, a compound of intense pharmaceutical interest ( Dai et al., 2016) and long-term traditional use ( Chen et al., 2009).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.