Jassavalida ( Dana, 1853 )
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4939.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:F33F42D0-A139-4CE3-97D7-1314C12CF86B |
|
DOI |
https://doi.org/10.5281/zenodo.4580564 |
|
persistent identifier |
https://treatment.plazi.org/id/03B487DA-FF8A-D950-C9C8-1DD6FBC3F888 |
|
treatment provided by |
Plazi |
|
scientific name |
Jassavalida ( Dana, 1853 ) |
| status |
|
Jassavalida ( Dana, 1853) View in CoL
( Table 10 View TABLE 10 , Figs 31–36 View FIGURE 31 View FIGURE 32 View FIGURE 33 View FIGURE 34 View FIGURE 35 View FIGURE 36 )
Cratophium validum Dana, 1853, pp. 841–843 View in CoL , plate 56, fig. 2
Podocerus validus ( Dana, 1853) : Bate (1862), p. 253, plate XLIII, fig. 9; Stebbing (1888), pp. 1135–1136, plate CXXXVIII. B; not Jassa pulchella Leach (1814) View in CoL : Stebbing (1906), p. 654
Jassa View in CoL sp. A: LeCroy (2007), pp. 565, 567, fig. 486.
Jassa mendozai Winfield et al., 2021 View in CoL
Diagnosis.
Both sexes:
Mandibular palp: article 2, dorsal margin without a fringe of setae.
Maxilla 1: without a seta or setal cluster at the base of the palp article 1.
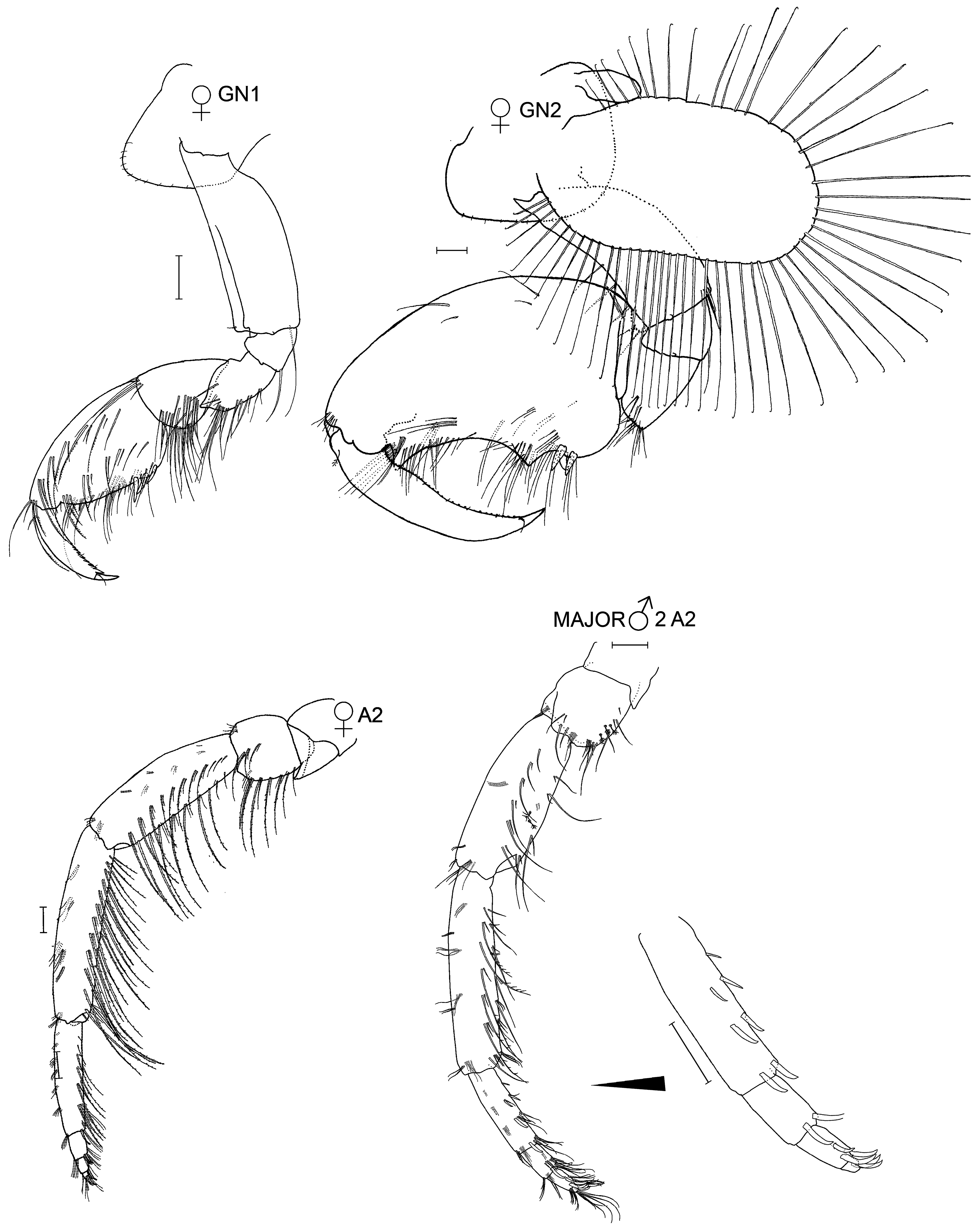
Gnathopod 1: basis, anterolateral margin with one distal and a few very short setae proximally; carpus with a single short, slightly medial seta at the anterodistal junction of the propodus (setal length <20% of the length of the anterior margin of the carpus).
Gnathopod 2: basis with a few setae along the anterolateral margin but without long filter setae (most setal lengths ± 40% of the basis width); carpus and propodus, setae on the anterior margin short and simple (setal length << basis width).
Pereopods 5–7: propodus not expanded anteriorly.
Uropod 1: ventral peduncular spinous process underlying about 1/2 of the longest ramus.
Uropod 3: inner ramus without spines mid-dorsally (with only the single apical spine).
Telson: tip with apical setae in addition to the usual short setae at each dorsolateral cusp.
Thumbed male:
Antenna 2: without plumose setae on the flagellum and peduncular article 5.
Gnathopod 2: propodus, palmar defining spines absent except in small males. Thumb distally acute in major and minor males. Dactyl expansion low, shallowly expanded proximally.
Adult female:
Antenna 2: without plumose setae on the flagellum and peduncular article 5.
Gnathopod 2: propodus, palm concave, palmar defining angle acute.
Description of the adult male, major form. Neotype (here designated). Length 5.5 mm.
Antenna 1: overlaps antenna 2 to about ½ peduncular article 5.
Antenna 2: article 5, posterior margin bearing short setae, these shorter than in the juvenile; flagellum 4 articles, article 1 65% of full flagellum length, posterior margin with pairs of spines on each article, 4 pairs on article 1 and 1 pair for each article thereafter at the posterodistal junction with the following article.
Mandible: palp articles 2 and 3 without dorsal fringe of setae; raker spines 2 right, 3 left.
Maxilla 1: palp without setae at based of article 1, article 2 with 1 row of facial setae.
Gnathopod 1: coxa strongly produced anterodistally, anterior margin 77% of dorsal length, ventral margin shallowly rippled; basis, anterior margin without a fringe of short, spine-like setae, posterior margin with 1 single cluster at junction of ischium; carpus, posterior lobe 37% of anterior margin length, with a single short seta medially at the anterodistal junction with the propodus; propodus, palm straight; dactyl cusped along most of posterior margin, without facial striations.
Gnathopod 2: coxal margins, anterior 39% and posterior 54% of ventral length, ventral margin straight; gill moderately large; basis, anterolateral flange with 5 widely spaced, relatively short setae (setal length ± 50% width of basis), palmar setae concentrated in hinge tooth area, thumb 39% of propodus length, distally acute, posterior margin shallowly concave, giving a conical appearance overall, defining setae closely approximated, not accompanied by spines; dactyl, inner margin shallowly expanded at the location of hinge tooth.
Pereopod 3: coxa, greatest depth posteriorly; basis, anterior margin shallowly concave; merus, anterior marginal setal clusters well separated, with 1 or 2 setae in middle clusters, setal length about 65% of article width; article width 60% of length; carpus fully overlapped by merus; propodus, width 55% of length.
Pereopod 4: coxa nearly rectangular, deeper than wide, ventrally convex; other articles as for pereopod 3.
Pereopods 5–7: propodus not expanded anteriorly, dactyl without fringe of setae along anterior margin, without facial striations.
Pleopods: with 2 peduncular coupling hooks.
Uropod 1: peduncle, posteroventral spinous process underlying 41% of inner ramus, inner and outer rami with 4 and 5 mid-dorsal spines, respectively, not terminating in a fringe of cusps ventral to apical spine group.
Uropod 2: peduncle, posteroventral spinous process underlying 15% of inner ramus.
Uropod 3: outer ramus with 2 sequential cusps proximal to the basally immersed, dorsally recurved spine and single seta originating at insertion of recurved spine; inner ramus with only single apical spine.
Telson: with 5 long, plumose setae at the apex in addition to the usual pair of short setae and single long seta at each lateral knob.
Condition. Without right antenna 1 and pereopods 5–7. Other right appendages, left antenna 1, pereopods 5–7 and uropod 3, telson and mouthparts slide mounted. Tip of right gnathopod 2 thumb and dactyl damaged, shapes drawn from the left gnathopod 2.
Description of the adult female. (Same location as for neotype, BBwB3). Length 3.6 mm. Character states as in the male except as follows.
Antenna 2: article 5, filter setae longer than in the neotype, as long and as dense as the filter setae on article 4.
Gnathopod 1: coxa, not as anteriorly produced as in the neotype, ventral margin relatively straight.
Gnathopod 2: coxal margins, anterior 51% and posterior 86% of ventral length, ventral margin straight; propodus, hinge tooth pronounced, palmar setae moderately dense throughout but not so dense as to obscure the palm’s shape, palmar angle acute, distal, but close to defining spines; dactyl, inner margin slightly expanded at the hinge tooth, tip reaching the palmar defining spines.
Telson: with 2 apical setae.
Condition. Brood plates setose, ovigerous. Without right pereopods 5–7, part of left antenna 2 and left pereopods 6–7. Other right appendages, left pereopod 5 and uropod 3, telson and mouthparts slide mounted.
Variation. Females and juvenile and subadult males have long filter setae on the posterior margin of antenna 2 peduncle article 5 while these shorten in thumbed males. The spination of the flagellum also varies, increasing in number on the flagellum article 1 in thumbed males, with a pair of spines on each additional flagellum article. In females and juvenile males, flagellum article 1 has a single distal pair on this article, followed by single pairs on successive articles. Subadult and minor form males are intermediate between the juveniles and thumbed males with more pairs of spines on flagellum article 1 than juveniles but fewer than the major form thumbed male. Subadult males also show a “pre-thumb” on the second gnathopod propodus distal to the palmar defining spines at the location of the palmar defining angle in the juveniles. Major form thumbed males lose the defining spines while minor forms retain them. The dactyl is also centrally expanded into the palm in minor forms. The number of setae on the gnathopod 2 basis varies from 5–6 but they are always widely spaced. The bases of pereopods 3 and 4, which hold the tube spinning glands are expanded in the females and juveniles but more slender in the thumbed males. The uropod 3 outer ramus can have 3 cusps proximal to the dorsally recurved spine rather than the usual 2. The number of apical setae on the telson varies from 1 to 5.
Type material. Neotype: major form male, JSIAH327 , ( Museu Nacional de Rio de Janeiro), catalogue no. MN- RJcarcino 029820, accession no. BMFVF063-19 , Brazil: Santa Catarina: Babitonga (26.239°S, 48.647°W), 5 Sept. 2017, from settling plate, 1.5 m depth, A. Desiderato, collector. GoogleMaps
Adult female, 3.6 mm, BBwB3, (Museu Nacional de Rio de Janeiro), catalogue no. MNRJcarcino 029821, Babitonga, Brazil (26.239°S, 48.647°W), 5 Sept. 2017, from settling plate, 1.5 m depth, A. Desiderato, collector GoogleMaps .
Juvenile male, 3.3 mm, JSIAH330 , ( Museu de Zoologia da Universidade Estadual de Campinas “ Ad „o José Cardoso ”) catalogue no. ZUEC CRU 4341 , accession no. BMFVF063-19 , Babitonga, Brazil (26.239°S, 48.647°W), 5 Sept. 2017, from settling plate, 1.5 m depth, A. Desiderato, collector GoogleMaps .
Subadult male, 5.6 mm, JSIAH323 , ( Museu de Zoologia da Universidade Estadual de Campinas “ Ad „o José Cardoso ”) catalogue no. ZUEC CRU 4342 , accession no. BMFVF064-19 , Babitonga, Brazil (26.239°S, 48.647°W), 5 Sept. 2017, from settling plate, 1.5 m depth, A. Desiderato, collector GoogleMaps .
Other material examined. 562 specimens from Brazil (Cananeia (25.008°S, 47.923°W and 25°02ʹS, 47°56ʹW), GoogleMaps Babitonga (26.239°S, 48.647°W), GoogleMaps Paranaguà (25.517°S, 48.501°W), GoogleMaps Pontal do Sul (25.559°S, 48.341°W), GoogleMaps Antonina (25.435°S, 48.705°W), GoogleMaps Guaratuba (25.873°S, 48.579°W), GoogleMaps Guaraqueçaba (25.435°S, 48.705°W), GoogleMaps Sao Paulo region (23°48ʹS, 48°34ʹW), GoogleMaps west of Chile ( Challenger station 302 (42°43ʹS, 82°11ʹW )); GoogleMaps Mississippi : Gulf coast; Florida : St. Andrews Bay , Panacea , Fort Myers , off Panama City , St. Augustine ; Georgia : Jekyll I.; North Carolina : Beaufort , Radio I., Fort Macon , Morehead City ( CMN, NHM, L. Pequegnat, Y. Wakabara). GoogleMaps
Remarks. Jassa valida was first described from a dredge collection in the harbour of Rio de Janeiro, Brazil during the United States Exploring Expedition led by Charles Wilkes (1838–1842) ( Fig. 7 View FIGURE 7 ). Dana (1853) originally named it Cratophium validum but it was subsequently transferred to Podocerus validus and then synonymized with Jassa pulchella (now J. falcata ) ( Stebbing 1906). As noted in Conlan (1990), the type specimens have been lost (confirmed absent from the collection of the Smithsonian Institution, National Museum of Natural History, 13 May 2019 by Karen Reed, Museum Specialist, Department of Invertebrate Zoology).
Dana’s (1853) illustrations of the adult male and female show three characteristics that, in combination, are unique to J. valida : apical telson setae, conical thumb and a short, medial seta at the anterodistal junction of carpus and propodus on gnathopod 1. Jassa valida shows the typical divergence of major and minor form thumbed males ( Fig. 34 View FIGURE 34 ) with the major forms having a much longer thumb than minors of the same body size but little overlap of the two in the population sampled. Subadult males with a thumbed cuticle visible inside the existing cuticle, had a small “pre-thumb”.
Thomson (1883) recorded J. valida in the harbour of Rio de Janeiro living in “...great numbers hiding among the hairs which line the sutures on the thoracic sterna of the common cray-fish ( Palinurus )...”. Most of these were females. Stebbing (1888) illustrated an adult male specimen collected off the coast of Chile at the ocean surface during the Challenger Expedition (station 302, 42°43ʹS, 82°11ʹW, 28 Dec. 1875). He showed (and described) apical setae on the telson, widely spaced setae on the basis of gnathopod 2 and a conical thumb, all key characters of J. valida . Further examination of this specimen for this study showed that the diagnostic seta on the carpus of gnathopod 1 was short and medial, another key character that identifies J. valida . Although this Chilean location is far from the type locality of Rio de Janeiro, it is possible that the Challenger was colonized by J. valida while the ship was previously in port in Valparaiso in Nov. 1875. Prior to that, the Challenger had been in Japan. Jassa valida is not currently known from either of these locations, though. Stebbing (1888) further mentioned that another specimen, which also appeared to be this species had been collected at the ocean surface and labelled “ Philippines, off Tablas”. This specimen was not figured or described and since the identification is unclear, it has not been mapped. Its collection would have been around Nov. 1874 prior to the Challenger sailing northward to Hong Kong.
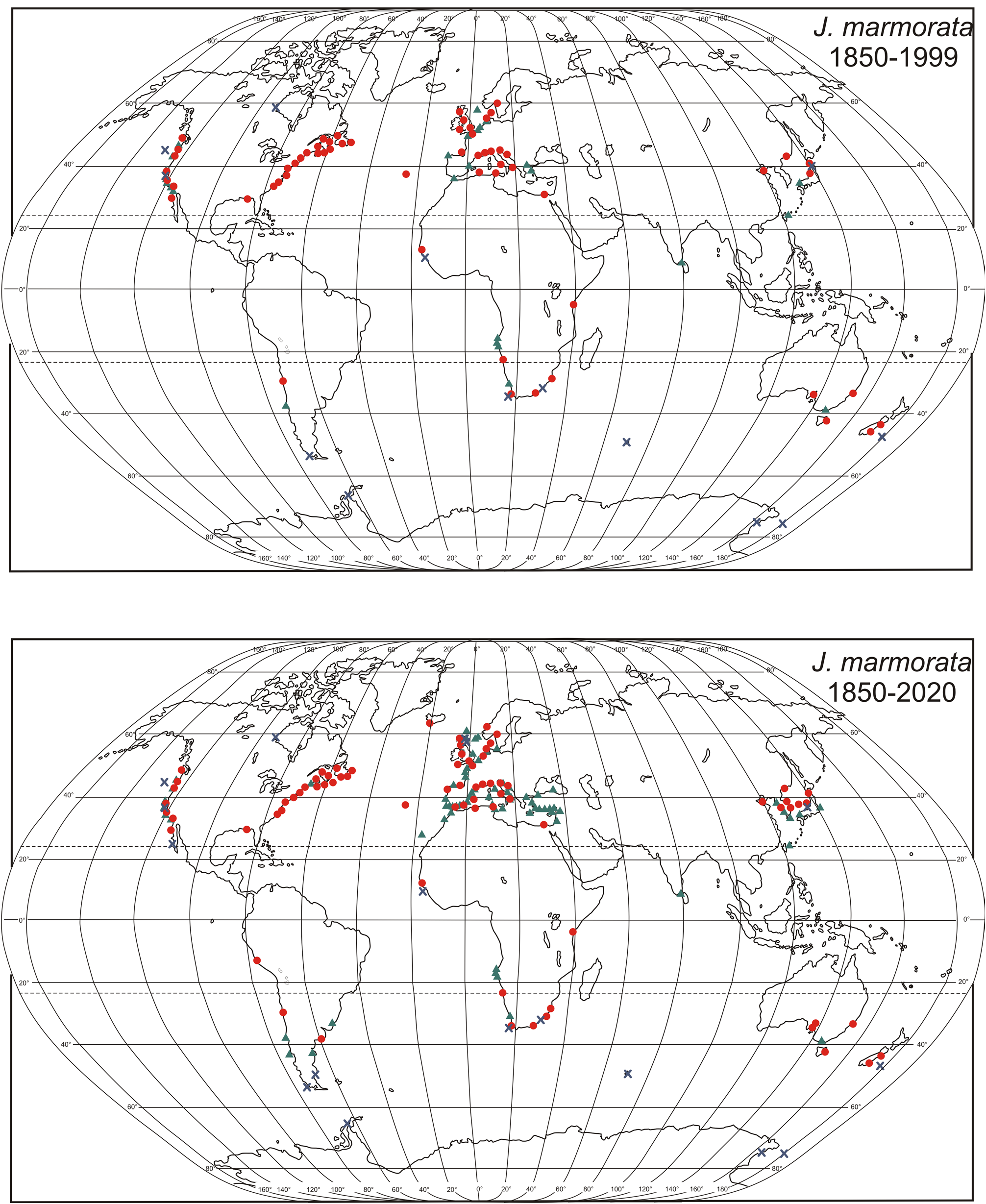
Specimens collected from settling plates at seven localities along the Brazilian coast in 2017 by one of us (AD) were found to be morphologically identical to Jassa valida . CO1 analysis further established them as being identical to a haplotype from Charleston Harbor, South Carolina, which was distinguished by Pilgrim and Darling (2010) as distinct from J. marmorata . This extended the known range of J. valida from Brazil to the U.S. Atlantic coast. Morphologically identical specimens illustrated by LeCroy (2007) as Jassa sp. Aextended the North American range of J. valida into the northern Gulf of Mexico. A recent collection in the southwestern Gulf of Mexico by Winfield et al. (2021), and reported as a new species ( J. mendozai ) is unmistakeably J. valida (Supplementary Data File S1). Finally, morphological re-examination by one of us (KC) of 338 lots in the international collection of the Canadian Museum of Nature (CMN), comprising about 5,700 specimens of species that could co-occur with or be confused with J. valida ( J. marmorata , J. slatteryi and J. morinoi ) showed that it had been mis-identified by Conlan (1990) as J. marmorata . The re-examination showed that Atlantic North American collections from North Carolina to Florida and into the Gulf of Mexico were more likely to be J. valida than J. marmorata , although the latter could be found there infrequently ( Fig. 8 View FIGURE 8 ). The northerly range of J. valida ended at Beaufort, North Carolina; any specimens northwards, from Virginia to Newfoundland, were exclusively J. marmorata ( Fig. 2 View FIGURE 2 ). Therefore, records of “ J. marmorata ” in OBIS for South Carolina, Georgia, Florida and into the Gulf of Mexico should be considered to require confirmation as they may pertain to mis-identified J. valida . Collections in the Smithsonian Institution (NMNH) that were examined for Conlan (1990) were unfortunately not available for re-examination as this might have extended the range of J. valida into Cuba and the Bahamas. Where unpublished notes for Conlan (1990) described morphologies that are indicative of J. valida , these localities have been included in the maps and habitat data.
Records of “ J. marmorata ” in the four quadrants of the Gulf of Mexico by LeCroy et al. (2009) and Paz-Ríos & Ardisson (2013) (and also listed in OBIS) may pertain to J. valida , though their stated wide distribution is based on literature forthe U.S. Gulf and Atlantic coasts, not Mexico. Amention of “ Jassa falcata ” collected from Laguna Madre, Mexico (western Gulf of Mexico) by Raz-Guzmán & Soto (2017) is possibly J. valida .
The J. valida collected by Winfield et al. (2021) from soft sediments at 456–3295 m depth in the southwestern Gulf of Mexico very possibly were not naturally at such great depth but were ship contaminants. No species of Jassa has been confirmed to exist at such great depth and Jassa is known to contaminate seawater systems in ships and aquaria (see the Distribution section for transhemispheric species). As a tube-liver on shallow-water solid substrates, J. valida does not have the morphology for deep living, such as eye and coxal reduction, longer and slenderer pereopods and weaker sexual selection of the second gnathopod than in shallower species ( Kodama & Kawamura 2019). Asimilar mis-interpretation of deep-sea collections by Kr̂ncke (1994, 1998) is reported by Sirenko (2004). Although the authors stated that a filter, placed midway along the length of the ship’s seawater system prevented individuals from reaching the sample sediments as they were sieved, this does not obviate the possibility that there was no pre-existing colony of Jassa living in the system. Inshore individuals could have accessed the ship’s seawater system while the ship was docked and established colonies there long prior to the cruise. Small hatchlings could have passed through the intercepting filter, allowing for individuals to establish colonies beyond the filter. Small numbers of individuals (29 J. valida and reported as a new species, along with five reported as “ J. marmorata ” and three as J. morinoi ” from 19 of 129 stations) could then have been dislodged over the 14 days of the cruise and been captured while the sediment samples were being sieved.
In South America, Alonso de Pina (2005) noted first records of J. marmorata on the coasts of Uruguay and Argentina but it has not been confirmed whether these were unrecognized J. valida . An effort to borrow these specimens was unsuccessful. In a comparative study of the amphipod fauna in Sargassum on two Itanhaém shores of Brazil, the “ J. falcata ” referred to by Wakabara et al. (1983) is likely J. valida . Aspecimen collected near Sao Paulo, Brazil for another study and lent by Y. Wakabara was J. valida . It has also been found with J. slatteryi in a scraping of the hull of a small ship in Brazil. Re-examination of CMN collections from the Pacific coasts of North and South America, Europe, Asia and Australasia did not yield J. valida . However, individuals sequenced from Durban, South Africa in 2019 (F. MacKay, coll.) and the French Frigate Shoals, northern Hawaiian Islands in 2007 ( Plaisance et al. 2011a, b) that are identical haplotypes to Brazilian and U.S. specimens suggest that J. valida is more widespread than currently recognized.
Jassa valida has been found among algae on non-natural substrates such as buoys, pilings, jetties, settling plates and offshore fish cages as well as on natural substrates such as rocks and floating Sargassum ( Table 4 View TABLE 4 ). These are typical habitats for any species of Jassa , but the CMN collections suggest that there can be habitat partitioning. On the Atlantic coast of North America, where its range overlaps with J. marmorata and J. pusilla , the three species may segregate by water mass ( Table 4 View TABLE 4 ). While J. valida and J. marmorata can both be found in shallow subtidal waters, J. pusilla occurs substantially deeper. A site-specific colonization study offshore of Panama City, Florida by Pequegnat and Pequegnat (1968) yielded “ J. falcata ” on submerged buoys at an inshore location 3.2 km (2 mi) from the coast and an offshore location 17.7 km (11 mi) from the coast. Examination of specimens lent from this study yielded only J. valida inshore and only J. marmorata offshore. The inshore location is not bathed by cooler offshore bottom water that the offshore location receives. This suggests that despite the range overlap, the two species do not co-occur in the same water mass. Jassa valida has been found mixed with J. marmorata in only two of the CMN collections, both from North Carolina, which is the southern end of the range of J. marmorata ( Fig. 2 View FIGURE 2 ) and the northern end of the range of J. valida ( Fig. 8 View FIGURE 8 ).
Jassa valida is consistently recognizable from J. marmorata and J. slatteryi by having the terminal setae on the telson at any age and in both sexes (which J. marmorata and J. slatteryi always lack) ( Table 10 View TABLE 10 ). Jassa morinoi and J. monodon also possess the terminal telson setae, but neither of these species have been found on the eastern coasts of North or South America. The presence, length and origin of the seta (or small cluster of setae) at the anterodistal junction of the gnathopod 1 carpus with the propodus is a useful character as it is present regardless of sex or age. In J. valida and J. marmorata , this seta (or small cluster of setae) is short, but differs in location. In J. valida it is slightly medial ( Fig. 31 View FIGURE 31 ) while in J. marmorata , it is slightly lateral ( Fig. 15 View FIGURE 15 ). In J. slatteryi and J. morinoi this seta is long ( Figs 22 View FIGURE 22 and 29 View FIGURE 29 ) while in J. monodon this seta is absent. The shorter and less dense setation on the basis of gnathopod 2 distinguish J. valida from J. marmorata and J. monodon , though not J. slatteryi and J. morinoi . Jassa valida never develops plumose setae on the distal end of antenna 2 as it ages and the pointed apex of the terminal male’s thumb distinguishes it from larger specimens of J. marmorata . The multiple spines on the antenna 2 of thumbed males of J. valida is distinctive although limited to this demographic. Jassa marmorata and J. monodon can achieve a greater size than J. valida , J. slatteryi and J. morinoi but summer specimens of J. marmorata can be as small so this is not a useful character trait. LeCroy (2007) also noted differences in living pigmentation between J. valida and J. marmorata but preservation can remove the pigmentation patterns.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Jassavalida ( Dana, 1853 )
| Conlan, Kathleen E., Desiderato, Andrea & Beermann, Jan 2021 |
Jassa mendozai
| Winfield 2021 |
Cratophium validum
| Dana 1853: 841 - 843 |
Jassa pulchella
| Leach 1814 |
Jassa
| Leach 1814 |