Cliona aff. schmidtii, sensu Schmidt, 1870
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4996.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:F398F5CE-82CA-48E2-98BA-9B59AF27DB5D |
|
persistent identifier |
https://treatment.plazi.org/id/292287D4-FF87-FF90-FF4B-F940FA8DC0B2 |
|
treatment provided by |
Plazi |
|
scientific name |
Cliona aff. schmidtii |
| status |
|
Cliona aff. schmidtii = Cliona schmidti sensu Ridley (1884) ≠ Cliona schmidtii sensu Schmidt (1870)
Synonymy. Vioa schmidti sensu Ridley (1884 ; full description of a specimen from the Seychelles erroneously identified as Cliona schmidtii ). Not: Vioa johnstonii Schmidt, 1862 , a Mediterranean Jaspis sp. ; Vioa johnstonii sensu Schmidt (1870 , p. 5 and pl. VI, fig. 18), the Mediterranean Cliona schmidtii ; Vioa schmidtii Ridley, 1881 (new species name to distinguish Schmidt’s 1870 purple Mediterranean Cliona from Schmidt’s 1862 Jaspis johnstonii ); Thoosa istriaca Müller, 1979 (sample combination of two species, containing Cliona cf. schmidtii ).
Material examined. Cliona aff. schmidtii —B1-PA-01, slide of discarded specimen kept at VUW, alphamorphology sponge from the channel between Kaledupa and Hoga, Wakatobi, Banda Sea, sampled between March and August 2014, 3– 20 m, coll. J. Marlow. B1-PA-02, slide of discarded specimen kept at VUW, alpha-morphology sponge from the channel between Kaledupa and Hoga, Wakatobi, Banda Sea, sampled between March and August 2014, 3– 20 m, coll. J. Marlow. B3-PA-01, slide of discarded specimen kept at VUW, alpha-morphology sponge from the channel between Kaledupa and Hoga, Wakatobi, Banda Sea, sampled between March and August 2014, 3– 20 m, coll. J. Marlow. BMNH 1882.10.17.159, Ridley’s (1884) slide preparation with spicules of an alpha-morphology sponge from Rémire, Amirante Isles, Seychelles, western Indian Ocean. CS-ORPH-b-13.1, slide of discarded specimen kept in CS’s personal collection, alpha-morphology sponge from Orpheus Island backreef, central Great Barrier Reef, Coral Sea, sampled 26. August 1996, 5 m, coll. C. Schönberg. Specimens of other species used in comparison were: Cliona jullieni — MNHN L.B.I.M. DT2520, slide with tissue fragments of Topsent’s (1891) holotype from La Reunion Island, western Indian Ocean. Cliona tinctoria — QM G313368, Schönberg’s (2000) holotype from Great Palm Island, central Great Barrier Reef, Coral Sea, sampled 7. December 1996, 3 m, coll. C. Schönberg, duplicate spicule slide in CS’s personal collection.
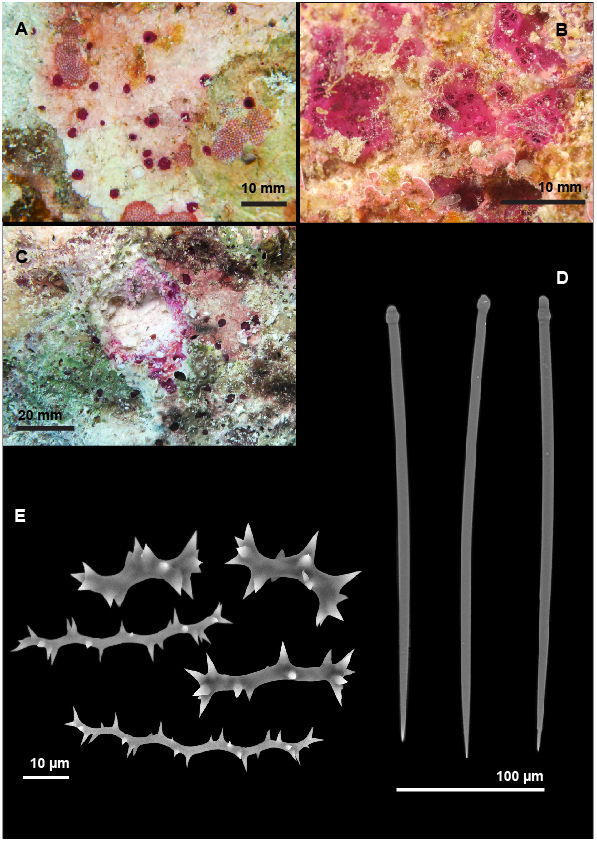
Morphology and erosion. Predominantly in alpha-morphology, with occasional tendencies to papillar fusion ( Fig. 5A View FIGURE 5 ). Papillae and choanosome vivid purple in situ, colour maintained after preservation in ethanol. Numerous circular papillae visible on substrate surface, usually up to 3 mm in diameter, but occasionally merging to form patches up to 10 mm in diameter including several papillae ( Fig. 5B View FIGURE 5 ). Inhalants level, fleshy patches with several minute pores. Exhalants of similar or larger diameter, conical, slightly raised above the substrate, with single oscular opening ( Fig. 5B View FIGURE 5 ). Short papillar canals (2–5 mm) connecting papillae to small erosion chambers (cross-sectional area 1.2 mm 2 ± 0.6 SD) in dense distribution and separated by irregularly shaped contiguous islands of substrate. Overall penetration depth rarely exceeding beyond 15 mm ( Fig. 5C View FIGURE 5 ).
Skeletal characteristics and presence of Symbiodiniaceae . Papillar tylostyles densely packed in palisade, with tips pointing to exterior. Tylostyles and abundant spirasters irregularly dispersed in papillar canals and choanosome. In situ low levels of fluorescence putatively indicative of photosynthetic symbionts, but no Symbiodiniaceae later observed within tissue.
Spicules. Megascleres—Straight to slightly curved tylostyles ( Fig. 5D View FIGURE 5 ), predominantly with spherical, but occasionally subterminal tyles, or as stylar modifications. Dimensions (min – mean – max and standard deviation): length 218 – 309.1 – 345 µm ± 25.9 SD; shaft width 5 – 6.6 – 9 µm ± 1.2 SD; and tyle width 5 – 8.5 – 12 µm ± 1.9 SD (means across three Wakatobi specimens, with N = 25 spicules each). Microscleres – Spirasters morphologically varied, apparently forming a continuum of lengths and widths, but broadly falling into two groups: 1) long and spindly spirasters with up to 7 slight bends and fine, discrete spines in regular spiral distribution on convex side of shaft ( Fig. 5E View FIGURE 5 ); and 2) shorter, stout spirasters with up to three bends and large conical spines, occasionally apically concentrated ( Fig. 5E View FIGURE 5 ). Dimensions of spindly variety 1 (min – mean – max and standard deviation): length 18 – 46.3 – 75 µm ± 15.6 SD; and width 1 – 2.3 – 4 µm ± 0.6 SD. Dimensions of stout variety 2 (min – mean – max and standard deviation): length 20 – 28.8 – 38 µm ± 4.9 SD; and width 3 – 5.4 – 10 µm ± 1.5 SD (means across three Wakatobi specimens).
Habitat and occurrence in the Wakatobi. Common; most frequently found in crevices on steep reef walls. Often associated with crustose coralline algae ( Fig. 5A View FIGURE 5 ), also commonly neighbouring and mingling with Cliothosa hancocki .
Remarks. Purple clionaid sponges visually stand out in the field and have been sampled from a wide range of sites, including:
• the Mediterranean, as Cliona schmidtii : e.g. Adriatic— von Lendenfeld (1898); Volz (1939); southern Tyrrhenian Sea— Rützler (1973); Balearic Sea— Rosell & Uriz (2002),
• the Atlantic, as Cliona schmidtii : Jamaica — Pang (1973); Belize — Rützler et al. (2014), and as Cliona carteri: Vitória-Trindade Ridge — Ridley (1881),
• the Indo-Pacific, as Cliona jullieni : e.g. Reunion — Topsent (1891); New Caledonia — Kelly-Borges & Vacelet (1998); Indonesia — Calcinai et al. (2017), and as Cliona schmidtii : e.g. Polynesia— Kirkpatrick (1900); Seychelles — Ridley (1884); Maldives — Calcinai et al. (2000); Indonesia —Hooper et al. (2000); Calcinai et al. (2017); Great Barrier Reef— Schönberg et al. (2006), as Cliona tinctoria : e.g. Great Barrier Reef— Schönberg (2000), and as Spheciospongia purpurea : e.g. southern coasts of Australia —e.g. de Lamarck (1814); Topsent (1906).
According to these accounts, there are presently only five valid species of purple clionaids ( Cliona carteri , Cliona jullieni , Cliona schmidtii , Cliona tinctoria and Spheciospongia purpurea ). Due to the comparatively distinct spicule characters and growth forms assigned to these names, their identification is not generally considered difficult. Based on spicule and skeleton characters Cliona jullieni , Cliona tinctoria and Spheciospongia purpurea were here readily determined as not being conspecific with the Wakatobi material, even though these species have been described from the Indo-Pacific. All three species possess small epidermal spirasters with irregular spine groups not found in the present samples. Moreover, their tylostyles are significantly more robust, and their mean tylostyle dimensions are considerably larger than those from the Wakatobi specimens (see Table 1). Spheciospongia purpurea can furthermore develop a massive morphology, which contrasts with the Wakatobi material that was predominantly found in papillate form.
Cliona carteri initially appeared similar to the purple sponge from Wakatobi, even though the two were found in different oceans. Ridley (1881) described the habit and the colour of the West Atlantic Cliona carteri as: “Vents scattered, papillary. Colour (in spirit) vivid crimson […] exactly the same as that of dry specimens of [ Cliona schmidtii …and] almost identical with that of [… Spheciospongia purpurea ]”. However, as for the Indo-Pacific species above, Cliona carteri has larger tylostyles of 394 x 15 µm, as well as only one kind of spirasters with a very slim axis (41 x 1 µm; Ridley 1881). We thus also excluded Cliona carteri from any further comparison.
The spicule morphology of the purple clionaid in the present study was a good match to that of the presently accepted holotype of Cliona schmidtii ( Ridley, 1881 —footnote on his p. 130). The type status is however erroneously designated to a sponge from the Amirantes that Ridley (1884) described as a “pink to crimson” sponge with papillae of 0.5 – 1.5 mm in diameter and tylostyles of 280 x 8 x 9.5 µm. Using Ridley’s spicule preparation, our own measurements produced a slimmer mean for tylostyle shaft width of the Amirante sponge than what Ridley had published and was in better agreement with the Wakatobi material ( Table 1). We therefore concluded that the Wakatobi sponges were conspecific with Ridley’s (1884) purple sponge from the western Indian Ocean. However, there are significant taxonomic issues, as Ridley (1881, 1884) meant to name Schmidt’s Adriatic purple clionaid. Misled by the “splendid violet colour”, Schmidt (1862, 1870) had regarded Jaspis johnstonii and Cliona schmidtii sensu stricto as conspecific (both as Vioa johnstonii ), arguing that the stellate microsclere in Jaspis and the spiraster in Cliona are the opposite extremes of a continuum ( Schmidt 1870, pp. 5-6). Ridley (1881, 1884) recognised Schmidt’s error and aimed to separate the two species by giving the purple clionaid a new name, Cliona schmidtii . However, by basing the description on the Amirante sponge instead of on the Adriatic one, Ridley yet again combined two distinct species in the new name. And these inhabited two different oceans. Consecutive publications did not question this perceived wide distribution (e.g. Rützler 1973; Rosell & Uriz 2002), and the problem became increasingly convoluted and confusing, e.g. by again using the same name for purple clionaids in the Caribbean (e.g. Pang 1973; Rützler et al. 2014). In order to resolve this tangled taxonomy, it would be necessary to designate a type for Schmidt’s original Adriatic sponge, which would represent Cliona schmidtii sensu stricto as originally intended by Ridley (1881). Any other purple clionaids that were published as Cliona schmidtii would then need to be biometrically assessed, and where possible genetically. Once the different species can be recognised and separated, they can be named and distinguished from Cliona schmidtii . Obviously, this task went far beyond the scope of this paper. Thus, despite finding the Wakatobi purple clionaid to be conspecific with Ridley’s Amirante sponge, we did not re-describe it under a new name, but instead used the name Cliona aff. schmidtii (see “Material examined” above).
A separation of the perceived species complex of purple clionaids is impossible without a large work effort, because the confusion has built over a long time, and earlier publications did not create morphologic data that can be compared. Previous accounts appear to have often relied on only cursory measurements of very few spicules, which may sometimes produce values that deviate from a mean obtained from a larger sample (see published versus measured values in Table 1). Moreover, not discerning between unfinished and fully formed spicules and not only relying on the latter can lead to a wide discrepancy of determined spicule dimensions between accounts, especially in terms of spicule widths ( Schönberg & Beuck 2007). Furthermore, it has never been properly investigated whether the spirasters in specimens of Cliona cf. schmidtii represent a size-continuum or size-classes, and if the latter case is true, how many classes need to be assessed. To date spiraster measurements for Cliona cf. schmidtii were conducted by picking a haphazard selection for slimmer and for thicker spirasters, and in consequence such subsamples may have varied from study to study. We were thus unable to compare spiraster dimensions between publications.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |