Harveyope Penz & DeVries, 2006
|
publication ID |
https://doi.org/ 10.5281/zenodo.2646145 |
|
publication LSID |
lsid:zoobank.org:pub:35C119EB-0729-41BF-A36B-E034E611CE38 |
|
persistent identifier |
https://treatment.plazi.org/id/03C487A2-FFAE-FFFC-FEFE-FC65DB1C5257 |
|
treatment provided by |
Plazi |
|
scientific name |
Harveyope Penz & DeVries |
| status |
gen. nov. |
Harveyope Penz & DeVries , NEW GENUS
Type species: Lemonias zerna Hewitson, 1872
Description ( Fig. 5 View FIGURE 5 )
Character state changes from our analysis are indicated by numbers in parentheses. Head: predominantly brown. Frons varying from brown ( zerna ) to mostly white ( glauca (Godman & Salvin)) ; white marking at center ranging from small (e.g., zerna ) to prominent ( glauca ), bordered in white or dull yellow ( densemaculata (Hewitson)) . Frons mostly covered with long hairlike scales projecting anteriorly. Head apex with long scales projecting anteriorly. Second segment of labial palpus laterally white or dull yellow ( densemaculata ), with long hairlike scales projecting ventrally; first and third segments brown. Antenna brown with white rings, club orange at tip. Body: dorsal body scales brown, ventral body and leg scales vary from white ( glauca ) to light brown ( densemaculata ). Wings: FW length 11–15 mm (n=15). Sexes similar. See Fig. 5 View FIGURE 5 for wing shape, venation, and location of spots and stripes. Dorsal wing color pattern: brown with darker brown spots ranging from conspicuous ( sejuncta (Stichel) , tinea ) to faded (e.g., zerna ), to nearly completely obliterated by colored scales ( glauca ). Green, blue or yellow lines crossing cell boundaries ranging from broad ( glauca ), to thin ( zerna, densemaculata ), or absent ( sejuncta, tinea ). Ventral wing color pattern: FW slightly paler than dorsal surface, brown spots always conspicuous, bordered or encircled with white to dull yellow scales. HW varies from being similar in color to ventral FW ( tinea, densemaculata ) to predominantly white with brown spots ( zerna, glauca ). Male terminalia: Sternite 8 extending posteriorly beyond the pleural membrane to form symmetrical, rounded, pointed or squared projections. Genitalic capsule varies from elongate to short and compact ( sejuncta, tinea ). Posterior edges of uncus straight and with nubs or spiny projections. Gnathos sickleshaped with a blunt tip. Vinculum not continuous through anterior edge of tegumen. Aedeagus (=phallus) with a long, acute tip. Coecum penis small or absent ( sejuncta, tinea ). Cornuti constituted of a simple plate (character 29:1), sometimes bearing spines ( sejuncta, tinea ). Vesica with prominent sculpturing (character 28:2). Valvae clearly divided into two processes; dorsal processes separated laterally, ventral processes forming a bridge above aedeagus. Tip of ventral process sometimes fused to this bridge ( sejuncta, tinea ). Lateral, rounded extension of ventral process protruding outward to form a small flap.
Remarks on female genitalia
The single female of the type species available to us had a broken abdomen, preventing examination of the genitalia. From examination of the female genitalia of glauca ( Fig. 5 View FIGURE 5 ) and tinea , we note the following: (1) antrum narrow and sclerotized in both species (but less sclerotized in tinea ), long in glauca and short in tinea ; (2) in glauca ductus bursa with a lightly sclerotized, internally spined enlargement before corpus bursa that seems identical to that found in Hallonympha paucipuncta (this structure is absent in tinea ); (3) corpus bursa highly elongated; (4) four signa in glauca , two in tinea . Differences in female genitalic morphology between glauca and tinea appear to be comparable to that in Hallonympha (this study) and Catocyclotis ( Penz & DeVries 2004) .
Natural history
Collectively, species in this genus range from Nicaragua south to Costa Rica, and in South America from Venezuela, Colombia, Peru and Brazil. Early stages of Harveyope species are unknown or undescribed. DeVries (1997) reported natural history observations for glauca and densemaculata .
Etymology We name this new genus for D. J. Harvey in recognition for his contribution to riodinid systematics.
Species in the genus
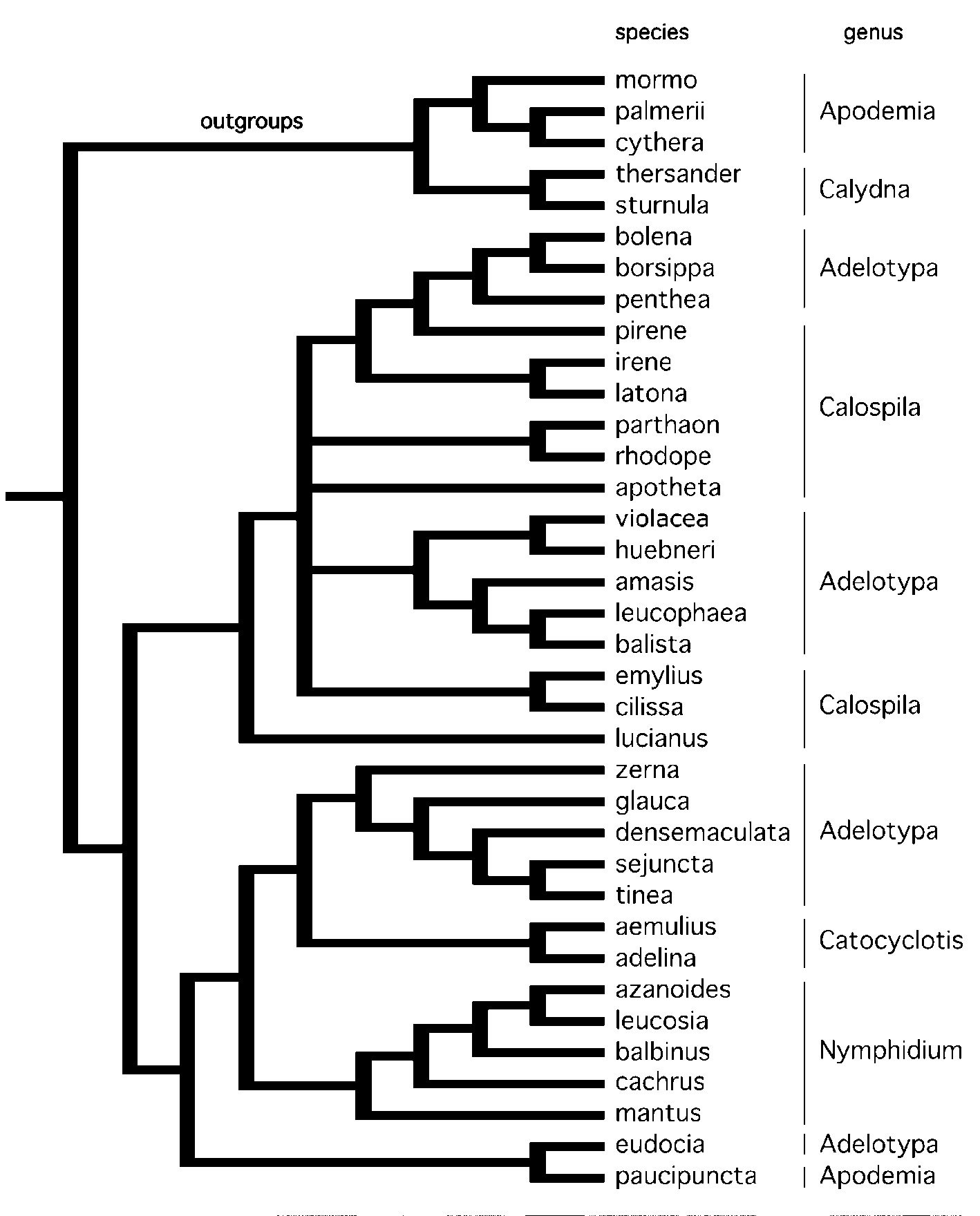
Based on our phylogenetic analysis ( Fig. 3 View FIGURE 3 ) and generic definition (above), we transfer the following species from Adelotypa to Harveyope :
Harveyope zerna (Hewiston, 1872) [type species], new combination
Harveyope glauca (Godman & Salvin, 1886) , new combination
Harveyope densemaculata (Hewitson, 1870) , new combination
Harveyope sejuncta ( Stichel, 1910) , new combination
Harveyope tinea (Bates, 1868) , new combination
Other species possibly included in Harveyope
We did not examine Adelotypa curulis (Hewitson, 1874) or Adelotypa argiella (Bates, 1868) . The male holotype of curulis (in the Natural History Museum, London; illustrated in D’Abrera 1994) appears similar to H. glauca . The specimen illustrated in D’Abrera (1994) as argiella resembles H. tinea . The possible phylogenetic relatedness among these taxa should be verified by future work.
Comparative morphology of the nymphidiine valva and transtilla
Regions of the valva
Based on the dytrisian groundplan ( Kristensen 2004 and references therein), we interpret the valva of species in our ingroup as having two recognizable regions that slightly twist around each other: (1) an outer region curved downward and extended towards the ventral portion of the valva, and (2) an inner region curved upward and extended towards the dorsal portion of the valva ( Fig. 6 View FIGURE ). Both ventral and dorsal regions bear a row of setae that can be used to help recognize their spatial orientation and degree of morphological modification. In the ingroup these regions are not sclerotized in their entirety, and a fissure is visible laterally on the valva. The valva regions can diverge from each other and give rise to distinguishable ventral and dorsal processes (e.g., Calospila lucianus (Fabricius) and Harveyope zerna , Fig. 6 View FIGURE ), or can merge to produce a simple tube (e.g., Adelotypa bolena , Fig. 6 View FIGURE ). The ventral and dorsal processes vary in length, width, degree of sclerotization, spatial orientation, and ornamentation. Illustrations in Fig. 6 View FIGURE will facilitate their identification and comparison. We stress that only through unequivocal identification of the valva processes as a frame of reference can meaningful comparisons across species be made. The following subsection illustrates this particularly well.
Fusion of the valvae and the nymphidiine transtilla
In Nymphidiini , the fusion of the valvae can be described as pertaining to two general categories of morphological modifications: (1) the longitudinal fusion of the valvae, or (2) the development of an outgrowth that forms a bridge between the valvae.
Either the dorsal or the ventral processes of the valva can be fused along their length, and produce unambiguously distinct morphological patterns. For example, the valvae of Calospila parthaon and Adelotypa bolena ( Fig. 6, 18 View FIGURE ) are fused dorsally along a large portion of their length by cuticle that is less sclerotized than the remainder of the valvae. In contrast, in species of Synargis (e.g., see S. abaris illustrated in Penz & DeVries 1999) the valvae are heavily fused ventrally along most of their length. Lateral movement of the valvae is compromised in both cases, and the dorsal or ventral fusions of the valvae likely have distinct effects on the mechanics of copulation. The difference in topological origin suggests that the dorsal and ventral fusions of the valvae evolved independently within Nymphidiini .
When the entire valvae are sufficiently separated from each other laterally, either the dorsal or the ventral processes may produce an outgrowth that bridges the two valvae above the aedeagus. Here the resulting morphological patterns can be misleadingly similar. A case in point is to compare the bridge formed by the dorsal process of the valva in Calospila lucianus with that formed by the ventral process in Harveyope zerna ( Fig. 6 View FIGURE ). Consideration of the different topological origin of these bridges suggests that they are not homologous (see Discussion). For this reason we used separate characters (42 and 48) to address the ‘dorsal’ and ‘ventral’ bridges identified in this study (Appendix 1).
In sum, within Nymphidiini we identified four distinct morphological modifications that limit the lateral movement of the valvae. From direct, comparative observation and careful consideration of published studies (e.g., Hall & Harvey 2002), we concluded that three of these modifications have been collectively called transtilla: the dorsal fusion of the valvae, plus the two different sclerotized bridges between the valvae described above. We therefore recommend that the term transtilla be replaced by language that precisely defines the structures found within Nymphidiini (e.g., characters 41, 42 and 48; Appendix 1). In this way information potentially useful for phylogeny reconstruction can be more rigorously assessed.
Discussion
Monophyly of Adelotypa and Calospila
Our initial quest for the generic position of Hallonympha paucipuncta became a broader venture than anticipated. Our preliminary intuition suggested that paucipuncta was a member of Adelotypa . However, we found that neither Adelotypa nor its relative Calospila were monophyletic. Although the purpose of our analysis was to provide a generic placement for paucipuncta , we also found it necessary to redefine male characters relevant to phylogenetic studies of Nymphidiini and to describe two new genera.
In the most recent attempt to sort Adelotypa into species groups, Stichel (1930) divided the genus (as Echenais ) into four groups ( Table 3 View TABLE 3 ): aristiformes (15 species), densemaculatiformes (5 species), pentheiformes (4 species), and sentiformes (5 species). Our analysis ( Figure 3 View FIGURE 3 ) does not fully confirm Stichel’s classification, as noted below:
(1) aristiformes: we found that this does not constitute a natural group, but includes members of at least four lineages — the amasis group, part of Harveyope , Hallonympha eudocia , and the notably divergent lampros that seems misplaced in Adelotypa and almost certainly represents a distinct genus;
(2) densemaculatiformes: this group contains three species in Harveyope , plus curulis and melitta (not examined by us). Stichel’s densemaculatiformes is therefore paraphyletic with respect to sejuncta and tinea ;
(3) pentheiformes: Adelotypa penthea was the only species within pentheiformes sampled here, and it grouped with bolena and borsippa . Judging by the large number of character state changes accumulated by penthea (Appendix 3), this species seems problematic and warrants further study;
(4) sentiformes: the type species of Adelotypa ( bolena ) plus borsippa were included by Stichel in the sentiformes, and in our analysis bolena and borsippa emerged as sister taxa, confirming Stichel’s grouping. The nominate species of this group, senta , was placed in the genus Protonymphidia ( Nymphidiini :Theopeina) by Hall (2000). In agreement with Penz & DeVries (2004), elpinice Godman was moved to Catocyclotis by Callaghan & Lamas (2004).
Although our analysis did not include the whole of Adelotypa , it confirmed the suggestion that the genus is polyphyletic ( Hall 2000), thus establishing a framework for future studies.
The genus Calospila is thought to include 33 species ( Callaghan & Lamas 2004), and acknowledged to be in need of revision ( DeVries 1997). We examined a small fraction of Calospila , but all species grouped with various Adelotypa in our trees ( Fig. 2 View FIGURE 2 and 3 View FIGURE 3 ). While our sample size is insufficient to justify taxonomic changes, our study provides evidence that Calospila is not monophyletic. Finally, based on our analysis it is evident that Adelotypa and Calospila have intertwined evolutionary histories and that future phylogenetic studies should examine species representing both genera.
a currently in the genus Protonymphidia ( Hall 2000) . b based strictly on our analysis. Additional Nymphidiini genera are needed to verify these suggestions. c currently in the genus Catocyclotis ( Penz & DeVries 2004, Callaghan & Lamas 2004).
Tracing characters of special interest
Here we illustrate characters of special interest traced onto the SAW tree ( Fig. 3 View FIGURE 3 ). A complete list of character state changes for all branches of this tree is provided in Appendix 3. The aim of the discussion below is to complement the characters listed in Table 2 View TABLE 2 that define Hallonympha and Harveyope , and to summarize our viewpoint on particular characters currently used in the systematics of Nymphidiini .
Character 1 ( Fig. 7 View FIGURE 7 ). Location of 3 rd abdominal spiracle [in male adult]: above midline (0), at midline (1), below midline (2).
The original formulation of this character ( Harvey 1987) included two character states: dorsal (closer to tergite than sternite), and ventral (closer to sternite than tergite). The latter character state was considered diagnostic of, and universal to Nymphidiini (as defined by Harvey 1987), and correlated with the ventral position of larval spiracles on A3–7.
We found scoring the position of the 3 rd abdominal spiracle was not completely straightforward. Our measurements indicated that it is located clearly above or below the midline in some species, but in others it is located at midline ( Fig. 7 View FIGURE 7 ). In our sample most of the variation in this character was confined to the group including Nymphidium , Catocyclotis and Harveyope , and the A3 spiracle is dorsal in Hallonympha and three of five species of Harveyope . Of further interest is that in the larva of Catocyclotis adelina the A3–7 spiracles are dorsal ( DeVries et al. 2004), yet the adult A3 spiracle position does not seem to correspond with that of the larva. In sum, the variation in spiracle position strongly suggests that comparative measurements of many more species are needed to improve our understanding of this character and its ontogenetic correlates.
Character 3 ( Fig. 8 View FIGURE 8 ). Stn8 with terminal projection extending beyond edge of pleural membrane (0), devoid of such projection (1); and
Character 4 ( Fig. 8 View FIGURE 8 ). Stn8: simple, not divided (0), divided into two symmetrical projections (1), divided into asymmetrical projections (2).
Harvey (1987) defined his Lemoniini based on the presence of ‘bifurcated rami.’ Due to its highly diverse morphology and clear possession by several taxa of Harvey’s Nymphidiini , this structure was reinterpreted by Penz & DeVries (1999) who used ten characters to describe the range of variation in 15 genera. Subsequently Hall & Harvey (2002) used two characters to describe variation in the ‘bifurcated rami’ in an analysis including 11 species in 11 genera, and suggested that this structure probably evolved multiple times within Nymphidiini .
Our observations suggest that abdominal projections seem to be extraordinarily plastic ( Penz & DeVries 1999, this study). In the context of out and ingroups analyzed here, abdominal projections have been lost in apotheta and lucianus ( Fig. 8 View FIGURE 8 ), while both simple projections in bolena + borsippa and the amasis group, and the asymmetrical projections of Nymphidium appear to be derived from ancestral divided, symmetrical projections (i.e., bifurcated rami sensu Harvey). These patterns suggest that these structures might evolve in any given direction. Even with our limited taxon sampling it seems evident that morphological divergence is unevenly distributed on the tree, and that some groups (e.g., amasis group) are less variable than others (e.g., Catocyclotis , see illustrations in Penz & DeVries 2004). Although the projections of the last abdominal sternite may be useful for species level associations, marked differences in morphology, in concert with apparently uneven rates of divergence, may limit their utility in higherlevel classification ( Penz & DeVries 1999, Hall & Harvey 2001, 2002).
Character 24 ( Fig. 9 View FIGURE 9 ). Vinculum: continuous through entire anterior edge of tegumen (0), not continuous through entire anterior edge of tegumen (1).
This character was first defined by Penz & DeVries (1999) and used to group Nymphidium and Theope . Hall & Harvey (2002) later termed it ‘incomplete vinculum’ and used it to define their Theopeina, with the caveat that Nymphidium and Catocyclotis (in their Nymphidiina) also possess an incomplete vinculum. Here we show that character state 24:1 is a synapomorphy for Hallonympha + Nymphidium + Catocyclotis + Harveyope and it also appears in the distant Apodemia mormo (C. Felder & R. Felder) , A. palmerii (Edwards) and A. cythera (Edwards) (plus A. walkeri Godman & Salvin and A. nais (Edwards) , not included in our analysis). This character state is therefore more ubiquitous than recognized previously, and its value for subtribal classification requires reassessment through analysis of a broader range of taxa than presently available.
Character 29 ( Fig. 9 View FIGURE 9 ). Cornuti: absent (0); a simple plate (1); a plate with one terminal spine (2); a plate with two terminal spines of uneven size (3); a plate with multiple terminal spines of approximately even size (4); two spines (5); multiple spines, short, thick, heavily sclerotized (6); multiple spines, long, thin, lightly sclerotized (7).
Cornuti were highly variable within the species sampled here; we identified seven character states. Tracing these onto the tree illustrates that while it is conserved in certain taxa (e.g., amasis group, lucianus and emylius + cylissa) it can be variable within others (e.g., Harveyope , Catocyclotis ). Furthermore, except for Harveyope where a ‘plate with multiple spines’ seems to have arisen from a ‘spineless plate,’ none of the other character state changes represent intuitive morphological transitions. In sum, although the cornuti can provide phylogenetic information useful for some specieslevel groupings, high plasticity may limit their utility for higherlevel groupings within Nymphidiini .
Character 39 ( Fig. 10 View FIGURE 10 ). Valva: inner and outer regions forming dorsal and ventral processes that are visibly separated (0); inner and outer regions merging to form a single piece (1); and
Character 40 ( Fig. 10 View FIGURE 10 ). Dorsal process of valva extends posteriorly beyond ventral process (0), ventral process extends posteriorly beyond dorsal process (1).
When considered together, characters 39 and 40 can be used to propose a hypothesis about the evolution of valva morphology in Adelotypa bolena and borsippa and Calospila penthea and pirene (Godman). The valva of these species can be described as a laterally compressed tube that terminates in a single terminal point. Both rows of setae that serve as landmarks of the dorsal and ventral processes converge toward this point (39:1 above; Fig. 20 View FIGURE 20 ).
However, due to the tubelike configuration found in bolena , borsippa , penthea and pirene, it was not possible for us to determine a priori if the valva point originated from the dorsal (40:0) or the ventral process (40:1). These species were thus scored ‘uncertain’ (‘?’) for character 40 (Appendix 2).
Despite being scored as ‘uncertain’ for character 40, bolena , borsippa , penthea and pirene fell within a species group where the ventral process of the valva extends posteriorly beyond the dorsal process (40:1; Fig. 10 View FIGURE 10 ). Thus, we hypothesize that the dorsal process is reduced in these species, and that the terminal point of their valva corresponds to the ventral process. The combined information from characters 39 and 40 appear to provide a means for understanding morphological evolution within one species lineage in this paper.
Character 41 ( Fig. 11 View FIGURE 11 ). Valva: dorsal processes closely adjacent to each other, fused or nearly so (0); separated (1); and
Character 48 ( Fig. 11 View FIGURE 11 ). Valva, when dorsal process separated: ventral process extended to form a sclerotized bridge (i.e., ventral bridge) (0); not extended to form a sclerotized bridge (1).
In some riodinid species where the entire valvae are sufficiently separated laterally (41:1), sclerotized bridges may join them above the aedeagus (48:0). Character state 48:0 clearly groups the new genera Hallonympha and Harveyope with Catocyclotis and Nymphidium , and the ‘ventral’ bridge present in species of these genera should not be confused with that produced by the dorsal process of the valva (42:0, state changes not illustrated), or with the dorsal fusion of the valvae (41:0).
Character 58 ( Fig. 12 View FIGURE 12 ). In ventral view, rounded extension of the outer region of the valva: protruding outward to form a ‘flap’ (0), not protruding outward (1).
Excepting eudocia , this ‘flap’ ( Fig. 20 View FIGURE 20 ) was present in all other species of Hallonympha , Nymphidium , Catocyclotis and Harveyope examined here. Because it is easily recognized and scored, and seems consistent within this group of genera (additional Nymphidium species need to be examined for confirmation), we believe this character may be useful and highly informative for phylogenetic studies of Nymphidiini .
Character 60 ( Fig. 12 View FIGURE 12 ). In ventral view, anterior edge of valvae straight (0), projected (1).
This is one of the characters setting Harveyope and Catocyclotis (60:1, Fig. 20 View FIGURE 20 ) apart from Hallonympha and other genera (60:0). In our analysis, although highly consistent within the ingroup, this character also occurs in the more distantly related outgroups, illustrating the difficulty in finding unique, universal characters to support genuslevel relationships within Riodinidae .
Character 63 ( Fig. 13 View FIGURE 13 ). Number of spots in ventral hindwing cell Sc+R1: none (0), 1 (1), 2 (2), 3 (3); and
Character 64 ( Fig. 13 View FIGURE 13 ). Number of spots in ventral hindwing cell Rs: none (0), 1 (1), 2 (2), 3 (3).
Characters 63 and 64 were adapted from Hall & Harvey (2002). Excepting Nymphidium , the number of spots in the ventral hindwing cell Sc+R1 appeared consistent across species (plesiomorphic in 2 of our taxa) and their position inside the cell varied among taxa. In contrast, the pattern of spots in the neighboring cell Rs was much more variable both in number and position (not illustrated). Based on the study by Hall & Harvey (2002) and tracing patterns of characters 63 and 64 ( Fig. 13 View FIGURE 13 ), we speculate that the ‘presence of wing spots’ may constitute an ancestral condition within Nymphidiini . In a way similar to Schwanwitsch’s nymphalid groundplan (see Nijhout 1991), it is possible that a ‘spotted’ configuration may contribute the pattern elements from which others have arisen.
Character 70 ( Fig. 14 View FIGURE 14 ). Second segment of labial palpus, long, erect hairlike scales projecting ventrally beyond the flattened scales: absent (0), present (1); and
Character 71 ( Fig. 14 View FIGURE 14 ). Frons scales: mixed broad and thin, converging from sides to midline (0); thin scales more prominent and long, erect (1).
The presence of projected hairlike scales in the palpi (70:1, homoplasious in our tree), and the long, projected scales on the frons (71:1, plesiomorphic in our tree) are shared by Hallonympha and Harveyope . Although other characters provided evidence for separating these two genera ( Table 2 View TABLE 2 ), we note that head morphology provides potential evidence to group them. In the context of our taxon sampling regime, placing Hallonympha as sister to Harveyope increases tree length by six steps. Nonetheless, the usefulness of characters 70 and 71 should be revisited in the context of a more comprehensive study.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Harveyope Penz & DeVries
| Penz, Carla M. & Devries, Philip J. 2006 |
Harveyope sejuncta ( Stichel, 1910 )
| Penz & Devries 2006 |
Harveyope tinea (Bates, 1868)
| Penz & Devries 2006 |