Goezeella mariae, Philippe V. Alves & Alain de Chambrier & José L. Luque & Tomáš Scholz, 2017
|
publication ID |
https://doi.org/ 10.5281/zenodo.893547 |
|
DOI |
https://doi.org/10.5281/zenodo.5672081 |
|
persistent identifier |
https://treatment.plazi.org/id/3B7D7060-FFB5-E777-FC70-2CEA2DC5F8BD |
|
treatment provided by |
Plazi |
|
scientific name |
Goezeella mariae |
| status |
sp. nov. |
Goezeella mariae sp. nov.
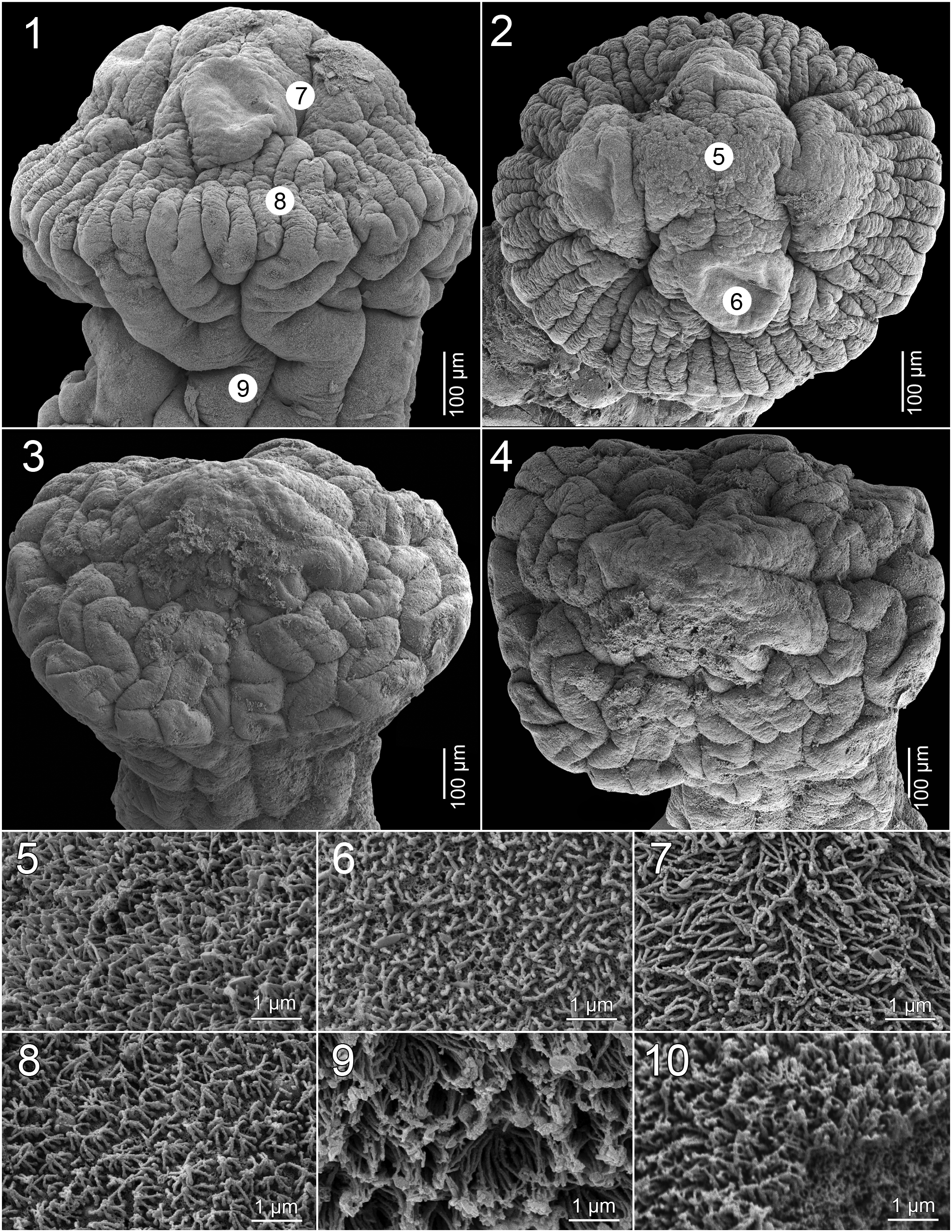
Figs 1, 2, 5-9 View Figs 1 - 10 , 22-30 View Figs 22 - 27 View Fig. 28 View Figs 29 - 30
Holotype: CHIOC 38860a-f, a whole-mounted specimen (1 slide) and 5 slides of serial cross-sections, collected on 25.05.2013, host field no. BR AMP 46a. – MHNG-PLAT-97017 (2 slides of cross-sections).
Paratypes: IPCAS C-759, a whole-mounted specimen (1 slide; hologenophore), host field no. BR AMP 106b. – CHIOC 38861, IPCAS C-759, MHNG-PLAT-86883, a whole-mounted specimen (one slide), 6 slides of serial cross-sections and 2 slides of sagittal sections of scolex, host field No. BR AMP 109a. – MHNG-PLAT-97016, a whole-mounted specimen (one slide; SEM voucher), host field no. BR AMP 111b; all specimens collected on 25.05.2013.
Type and only known locality: Lowermost Amazon River near Macapá, State of Amapá, Brazil (00°01’N, 50°59’W).
Type and only known host: Pimelodella cristata (Müller & Troschel) ( Siluriformes : Heptapteridae ).
Site of infection: Anterior intestine.
Prevalence: 7 fish examined/4 fish infected (57%).
Representative DNA sequences: A fragment 1491bp long of the lsr DNA (D1–D3 domains) (GenBank MF370208 View Materials ).
Etymology: The species is dedicated to the first author’s mother, Maria Thereza Vieira Pinto Alves, for providing continuous support for his studies.
Description: (based on 4 whole-mounted worms; 13 slides with serial cross-sections of mature proglottids and 2 slides with sagittal sections of 1 scolex; 1 scolex studied using SEM). Proteocephalidae . Testes, ovary, vitelline follicles and uterus cortical; small-sized worm. Total body length 14-38 mm (n = 3), maximum width up to 1.3 mm (n = 3). Strobila acraspedote, anapolytic, with longitudinal and transverse grooves, consisting of about 40-90 proglottids: 27-32 immature, 4-8 mature, 10-15 pregravid and 23-39 gravid. Immature and mature proglottids much wider than long (length: width ratio 0.17-0.35), pregravid proglottids markedly wider than long (length: width ratio 0.40-0.76) and gravid proglottids slightly wider than long to much longer than wide (length: width ratio 0.80-2.60).
Scolex 0.68-0.83 × 0.91-1.15 mm (n = 3), much wider than neck (proliferation zone), 0.83-1.20 × 0.75-0.81 mm, bearing 4 biloculate suckers, with loculi unequal in size; anterior loculus 158-161 (x = 160; n = 3) in diameter, posterior loculus 123-126 (x = 125; n = 3) in diameter; loculi separated by inconspicuous interlocular septum ( Figs 1, 2 View Figs 1 - 10 , 22, 23 View Figs 22 - 27 ). Metascolex present, more wrinkled than neck ( Figs 1, 2 View Figs 1 - 10 , 22 View Figs 22 - 27 ). Apex rounded, lacking apical organ, with few gland cells ( Figs 1, 2 View Figs 1 - 10 , 22, 23 View Figs 22 - 27 ). Apex of scolex, lumen of suckers, surface between suckers and base of metascolex covered with acicular filitriches, less dense on lumen of suckers ( Figs 5-8 View Figs 1 - 10 ); neck covered with capilliform filitriches ( Fig. 9 View Figs 1 - 10 ).
Inner longitudinal musculature well-developed, formed by numerous, individual muscle fibres not forming compact bundles, more concentrated laterally ( Figs 28- 30 View Fig. 28 View Figs 29 - 30 ). Osmoregulatory canals situated at same level of lateral-most testes, median to vitelline follicles, markedly sinuous ( Figs 24-26 View Figs 22 - 27 , 28-30 View Fig. 28 View Figs 29 - 30 ); ventral osmoregulatory canal wider than dorsal one ( Fig. 30 View Figs 29 - 30 ).
Testes numerous, spherical to oval, small, 34-46 in diameter, in 1 irregular layer, 103-167 (x = 134; n = 13) per mature proglottid ( Figs 24, 25 View Figs 22 - 27 ). Testes form 1 irregular field on dorsal side, less numerous alongside median line of proglottids (uterine stem), usually reaching laterally to osmoregulatory canals, dorsally overlapping cirrus-sac, vitelline follicles and sometimes ovary ( Figs 24-26 View Figs 22 - 27 , 28- 30 View Fig. 28 View Figs 29 - 30 ). Testes present also in gravid proglottids.
Vas deferens coiled, with loops forming elongate field reaching to, but not crossing, median line of proglottid ( Figs 24-26 View Figs 22 - 27 ). Cirrus-sac elongated to pear-shaped, thinwalled ( Figs 24-26 View Figs 22 - 27 , 28 View Fig. 28 ), 130-213 × 59-85 (n = 13), its length representing 11-24% (x = 17; n = 13) of proglottid width. Sperm duct (internal vas deferens) sinuous ( Figs 24-26 View Figs 22 - 27 , 28 View Fig. 28 ). Cirrus muscular, reaching up to 64% (n = 13) of cirrus-sac length. Common genital atrium narrow, deep ( Figs 24-26 View Figs 22 - 27 , 28 View Fig. 28 ). Genital pores alternating irregularly, markedly pre-equatorial, situated at 7-17% (x = 11; n = 13) of proglottid length from anterior margin ( Figs 24-26 View Figs 22 - 27 ).
Ovary with wide isthmus in medulla and two follicular, grape-like lobes penetrating to dorsal cortex; numerous dorsal outgrowths present ( Figs 24, 25 View Figs 22 - 27 , 30 View Figs 29 - 30 ). Length of ovary represents 21-31% (x = 26%; n = 13) of proglottid length, its width representing 57-77% (x = 66%; n = 13) of proglottid width ( Figs 24, 25 View Figs 22 - 27 ). Mehlis’ gland about 60- 138 in diameter, representing 8-11% of proglottid width (n = 13). Relative ovarian size, i.e., percentage of ovary surface to total surface of mature or pregravid proglottids (see de Chambrier et al., 2012), 10-15% (x = 12%; n = 13).
Vaginal canal slightly sinuous, surrounded by chromophilic cells, wider in terminal part (pars copulatrix vaginae); terminal vaginal sphincter present ( Figs 24-26 View Figs 22 - 27 ). Vagina anterior to cirrus-sac (n = 32). Vitelline follicles cortical, ventral, forming 2 long uninterrupted bands, occupying large triangular field, widened and confluent posteriorly at ovary level; lateral to lateral-most testes ( Figs 24-26 View Figs 22 - 27 , 29 View Figs 29 - 30 ). Length of bands represents 73-91% (x = 83%) and 72-91% (x = 83%) of length of proglottid on poral and aporal side, respectively (n = 13) ( Figs 24, 25 View Figs 22 - 27 ). Uterus cortical, with development of type 2 (see de Chambrier et al., 2004b, 2015b); uterine stem and diverticula (lateral uterine branches) in mature and pregravid proglottids lined with numerous chromophilic cells, extended much beyond branches ( Fig. 24 View Figs 22 - 27 ). Uterus with 14-25 lateral diverticula on each side ( Figs 24, 25 View Figs 22 - 27 ). Eggs oval, outer envelope 21-25 × 18-19, bilayered embryophore 19-20 × 13-15, oncosphere 9-10 × 11-12, embryonic hooks 5-6 long ( Fig. 27 View Figs 22 - 27 ).
Remarks: Goezeella mariae sp. nov. differs from G. siluri and G. danbrooksi in having fewer testes (103- 167 vs. 183-310 and 282-366 in G. danbrooksi and G. siluri , respectively) and inconspicuous interlocular septum (not obvious in SEM images; see Figs 1, 2 View Figs 1 - 10 ), rather than the septum conspicuous as in the two other species. The new taxon can be further distinguished from G. siluri by its smaller dimensions, such as the total body length (14-38 mm vs. 90-230 mm), scolex width (0.91-1.15 mm vs. 1.45-1.94 mm) and the length of the cirrus-sac (130-213 μm vs. 220-340 μm) as well as the appearance of the metascolex, which is more wrinkled in G. mariae sp. nov. compared to that of G. siluri ; compare Figs 1, 2 View Figs 1 - 10 with Figs 3, 4 View Figs 1 - 10 . Moreover, G. mariae sp. nov. possesses a terminal, rather than markedly subterminal, vaginal sphincter as it is in G. danbrooksi .
The new species differs in its sequence of the partial lsr DNA gene (D1–D3 domains) from that of G. siluri from P. pirinampu in 14 nucleotides, i.e. genetic divergence 0.9%. A phylogenetic analysis (data not shown) revealed both taxa clustered in a clade comprising also both known species of Gibsoniela Rego, 1984 , i.e. G. mandube ( Woodland, 1935) and G. meursaulti de Chambrier & Vaucher, 1999 , parasites of the auchenipterid catfish Ageneiosus inermis (Linnaeus, 1766) in the Neotropical Region, but interrelations within this lineage remain unresolved. Close relationship of species of Goezeella with those of the genus Gibsoniela is not evident based on their morphology, because they differ in the position of the internal organs in relation to the inner longitudinal musculature (previously used to distinguish individual subfamilies – see Rego, 1994), but also by the morphology of the scolex (no metascolex in the latter genus) and their suckers (biloculate in Goezeella vs.
triloculate in Gibsoniela ) ( Rego, 1984; de Chambrier & Vaucher, 1999).
To the best of our knowledge, this is the first parasite found in Pimelodella cristata . This heptapterid catfish was described from a tributary of the Branco River, Guyana ( Bockmann & Guazzelli, 2003) and is distributed throughout the Amazon River basin, inhabiting the sand bottom of creeks and rivers ( Reis & Lima, 2009). Proteocephalus bagri Holcman-Spector & Mañé-Garzón, 1988 and P. rhamdiae Holcman-Spector & Mañé-Garzón, 1988 , both from Rhamdia sapo (Valenciennes) [syn. of Rhamdia quelen (Quoy & Gaimard) ] in Uruguay, are the only other proteocephalids known from heptapterids in South America ( Holcman-Spector & Mañé-Garzón, 1988). In addition, Proteocephalus brooksi García-Prieto, Rodríguez & Pérez-Ponce de León, 1996 was described from Rhamdia guatemalensis (Günther) in Mexico by García-Prieto et al. (1996).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.