Girardia paucipunctata Hellmann & Leal-Zanchet
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4438.3.8 |
|
publication LSID |
lsid:zoobank.org:pub:6BB71AE3-6D5B-4C55-9BC0-8E853AC05928 |
|
DOI |
https://doi.org/10.5281/zenodo.5969695 |
|
persistent identifier |
https://treatment.plazi.org/id/B06687E3-D371-6C3F-85B0-0B67FEA5FF67 |
|
treatment provided by |
Plazi |
|
scientific name |
Girardia paucipunctata Hellmann & Leal-Zanchet |
| status |
|
Girardia paucipunctata Hellmann & Leal-Zanchet ; sp. nov.
Etymology: The name is composed of the Latin prefix paucus (a few) and the adjective punctatus (spotted), alluding to the slightly pigmented body.
Material examined. Holotype: MZUSP PL.2138: coll. R.L. Ferreira, 19 July 2012, “ Areias de Cima cave”, “ Areias system”, Iporanga , São Paulo, Brazil—sagittal sections on 13 slides.
Paratypes: collected by R.L. Ferreira on the same date and at the same sampling locality as the holotype. MZU PL. 00279: sagittal sections on 6 slides; MZU PL.00280: sagittal sections on 7 slides; MZU PL. 00281: transverse sections on 8 slides.
Type-locality: “ Areias de Cima cave”, “Areias system”, Iporanga, São Paulo, Brazil.
Distribution: Known only from the type-locality.
Diagnosis: Troglobitic Girardia paucipunctata is characterized by dorso-ventral testes, a wide and ovoid bulbar cavity, a smoothly inclined bursal canal, a short common ovovitelline duct and male and female atria separated by a constriction.
Description. External features. After fixation, pale brownish dorsal surface with scattered, fine pigmentation ( Fig. 14 View FIGURES 14–15 ). Ventral surface whitish ( Fig. 15 View FIGURES 14–15 ). Triangular head with moderately sized auricles and a pair of minute eyes; posterior tip rounded ( Figs. 14–15 View FIGURES 14–15 ). Body up to 5 mm long and 2 mm wide ( Table 2). Mouth located in the median or posterior third of the body and gonopore in the posterior third of the body ( Table 2; Fig. 15 View FIGURES 14–15 ).
Epidermis, cutaneous musculature and sensory organs. The epidermis is pierced by openings from rhabditogen glands producing xanthophil rhammites, as well as from glands producing an amorphous, cyanophil secretion ( Figs. 16, 21 View FIGURES 16–21 ). Cyanophil glands are more abundant in the anterior extremity of the body. In addition, slightly erythrophil glands with coarsely granular secretion concentrate their openings at the body margins as well as medially at the posterior tip of the body, whereas the anterior tip receives finely granular, xanthophil secretion. Body pigment granules located beneath the epidermis.
Cutaneous musculature consisting of four layers, viz. a thin subepithelial circular layer, followed by a thin longitudinal layer, an oblique layer with decussate fibers and a thicker layer of longitudinal muscle ( Fig. 16 View FIGURES 16–21 ). The cutaneous musculature is weakly developed, with the ventral musculature (8–10 µm thick) being slightly thicker than the dorsal musculature (4–5 µm thick), having about the same thickness as the epidermis, in the pre-pharyngeal region. The cutaneous musculature is thicker laterally than medially and becomes thicker at the anterior region of the body.
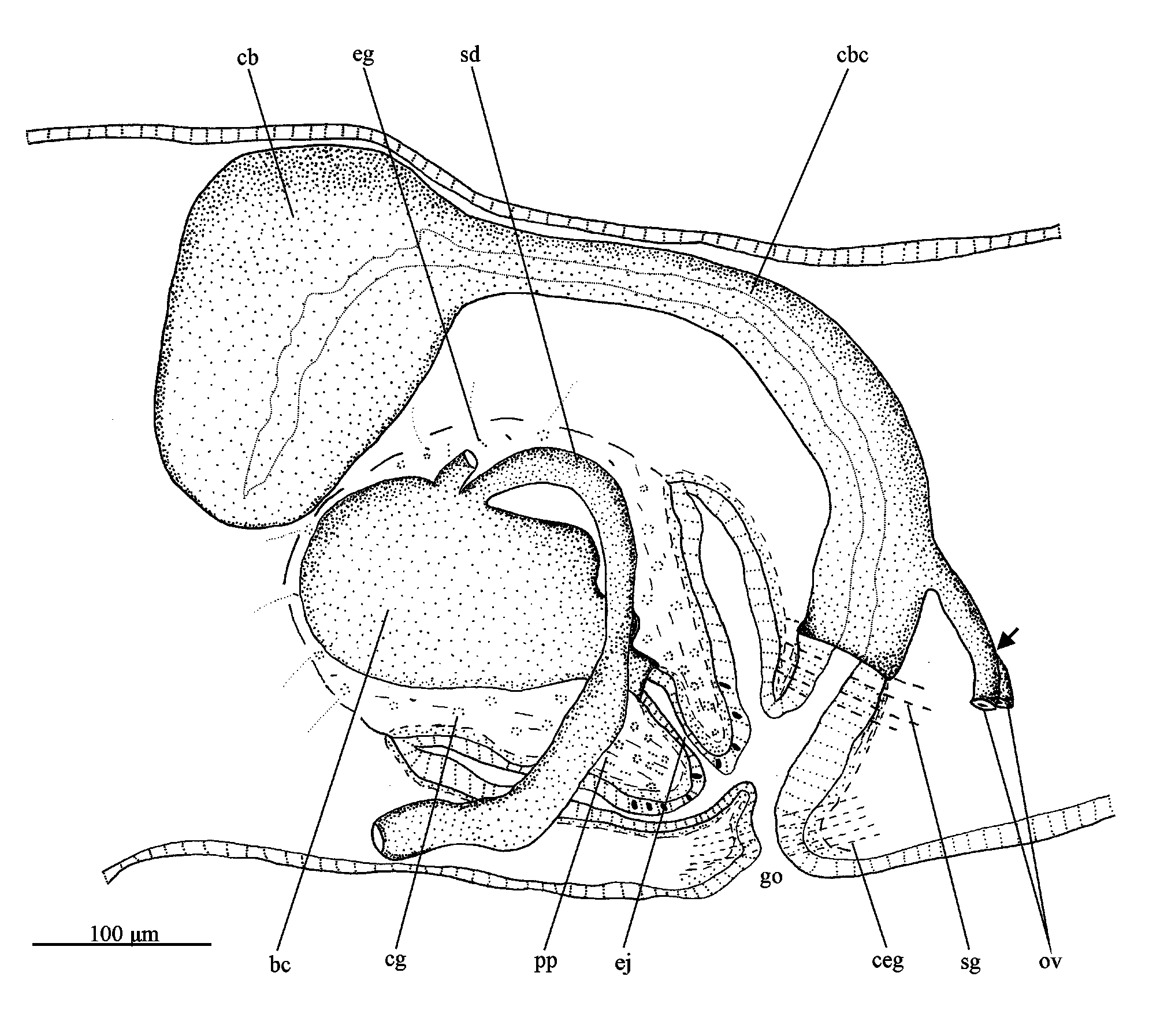
Digestive system. Pharynx cylindrical, pigmented; about 1/5th of the body length. It is located in the median third of the body. Mouth at the posterior end of the pharyngeal pouch. An esophagus, between about 1/9th of the pharyngeal length, connects the pharynx with the intestine. Anterior intestinal trunk extending dorsally to the brain. Male reproductive system ( Figs. 17–20 View FIGURES 16–21 , 22 View FIGURE 22 ). Numerous testicular follicles, about 70–90 µm in diameter, arranged in various irregular dorso-ventral rows on either side of the body ( Fig. 17 View FIGURES 16–21 ). Testes extend from about just behind the ovaries to the posterior end of the body. Sperm ducts form spermiducal vesicles posteriorly to the pharynx, which ascend in an almost straight course laterally to the penis. Subsequently, the ducts decrease in diameter and turn anteriad to separately penetrate the penis bulb. The sperm ducts open dorsolaterally into the wide and ovoid bulbar cavity ( Figs. 18 View FIGURES 16–21 , 22 View FIGURE 22 ). The short ejaculatory duct opens at the tip of the conical penis papilla ( Figs. 19 View FIGURES 16–21 , 22 View FIGURE 22 ). The penis papilla is obliquely oriented in the male atrium, being about 150 µm long and 200 µm wide at its basis.
Penial glands producing fine, slightly erythrophil granules (perhaps with a cyanophil external part) discharge into the bulbar cavity and ejaculatory duct. This secretion is also present in the bulbar cavity ( Figs. 18–20 View FIGURES 16–21 ). Xanthophil glands and cyanophil glands, both with amorphous secretions, and erythrophil glands with finely granular secretion, open through the epithelium of the penis papilla, whereas sparse glands with an amorphous, slightly cyanophil secretion open into the male atrium.
Female reproductive system ( Figs. 19–22 View FIGURES 16–21 View FIGURE 22 ). Vitellaria well developed in the holotype. Ovaries ovoid, about 130 µm in its lateral axis. The gonads are situated laterally to the ventral nerve cords, in close proximity of the brain, in the anterior 1/7th of the body. Ovovitelline ducts arise from the ventro-lateral surface of the ovaries, running backwards dorsally to the nerve cords, following a sinuous path. Behind the gonoduct, the ovovitelline ducts turn dorsally to join, more close to the ventral than to the dorsal epidermis, to form a short common section, which opens into the most distal part of the bursal canal ( Fig. 22 View FIGURE 22 ). Copulatory bursa ovoid, located anterodorsaly to the penis bulb ( Figs. 19 View FIGURES 16–21 , 22 View FIGURE 22 ). Bursal canal short, smoothly inclining ventrally to opening into the gonoduct, which is almost straight ( Figs. 20 View FIGURES 16–21 , 22 View FIGURE 22 ).
Copulatory bursa and bursal canal coated with interwoven longitudinal and circular muscles (about 2–5 µm thick). Bursal canal lined with a columnar, nucleated epithelium ( Fig. 21 View FIGURES 16–21 ). Xanthophil shell glands with finely granular secretion concentrate their openings into the most distal part of the canal, ventrally to the opening of the common ovovitelline duct. Cyanophil glands, as well as numerous cement glands, producing a coarsely granular, xanthophil secretion, discharge into the gonoduct ( Figs. 19, 20 View FIGURES 16–21 , 22 View FIGURE 22 ).
Comparative discussion. Regarding external features, besides differing from its epigean congeners, the trogobitic Girardia paucipunctata differs from most of other hypogean species by having a slightly pigmented dorsal surface and a pair of minute eyes ( Mitchell & Kawakatsu 1973b; Souza et al. 2015, 2016). Similar features are found only in the hypogean G. mckenziei ( Mitchell & Kawakatsu 1973a) .
With respect to the sympatric G. arenicola , herein described, mature specimens of G. paucipunctata have a smaller body and a weak pigmentation that is absent in G. arenicola . Regarding the pharynx, in G. arenicola it is non-pigmented and larger in relation to the body length, in contrast to the pigmented and smaller pharynx of G. paucipunctata . With respect to the reproductive system, G. paucipunctata shows overall similarities with G. arenicola , since both species have dorsal testes and a short common ovovitelline duct. However, G. paucipunctata can be easily differentiated from G. arenicola , since the latter has an almost horizontally disposed bursal canal, a bulbar cavity with multiple diverticula and sperm ducts opening laterally into the diverticula of the bulbar cavity. In contrast, G. paucipunctata has a smoothly curved bursal canal and an ovoid and unforked bulbar cavity, with the sperm ducts opening in their dorsolateral walls.
Among species of Girardia with dorsal or dorsoventral testes and a smoothly curved bursal canal, G. paucipunctata shares some similarities with G. antillana (Kenk, 1941) , G. arizonensis ( Kenk, 1975) , G. avertiginis Sluys, 2005 , G. barbarae (Mitchell & Kawakatsu, 1973) , G. capacivasa Sluys & Kawakatsu, 2005 , G. mckenziei and G. typhlomexicana ( Mitchell & Kawakatsu 1973a, b; Kenk 1975; Sluys 1992; Sluys et al. 2005). Similarly to these species, G. paucipunctata shows a wide bulbar cavity, while male and female atria separated from each other by a constriction.
Girardia paucipunctata can be distinguished from G. antillana View in CoL , G. barbarae View in CoL and G. capacivasa View in CoL by having a single bulbar cavity with the sperm ducts opening into this cavity without expansions. In contrast, G. antillana View in CoL shows a forked bulbar cavity with a blindly ending accessory chamber, G. barbarae View in CoL have a forked and elongated oval-shaped bulbar cavity and G. capacivasa View in CoL has large intrabulbar sections of the sperm ducts ( Mitchell & Kawakatsu 1973b; Sluys 1992; Sluys et al. 2005). Being unforked, the bulbar cavity of G. paucipunctata is similar to that of G. avertiginis View in CoL , G. arizonensis View in CoL and G. mckenziei View in CoL . However, in G. arizonensis View in CoL and G. mckenziei View in CoL , testes are absent behind the gonopore ( Mitchell & Kawakatsu 1973a; Kenk 1975), whereas in G. paucipunctata they extend to the posterior tip of the body. In addition, in G. mckenziei View in CoL , the sperm ducts traverse the penis bulb to open terminally into a short and wide lumen of the penis papilla ( Mitchell & Kawakatsu 1973a), differing from the situation in G. paucipunctata , since in this species the broad and ovoid bulbar cavity occupies the entire penis bulb and receives the sperm ducts dorsolaterally. The opening position of the sperm ducts into the bulbar cavity also distinguish G. paucipunctata from G. avertiginis View in CoL , since in the latter the sperm ducts open into the lateral wall of the bulbar cavity. In G. typhlomexicana View in CoL , the bulbar cavity may be forked in some specimens ( Mitchell & Kawakatsu 1973b), but G. paucipunctata differs from G. typhlomexicana View in CoL especially by having sperm ducts opening dorsolaterally into the bulbar cavity, whereas in G. typhlomexicana View in CoL the sperm ducts open terminally through the anterior wall of this cavity ( Mitchell & Kawakatsu 1973b). In addition, the bursal canal of G. paucipunctata , with intermingled muscles also distinguishes this species from G. antillana View in CoL , G. barbarae View in CoL , G. arizonensis View in CoL , G. mckenziei View in CoL and G. typhlomexicana View in CoL , which show circular subepithelial muscles followed by longitudinal muscles ( Kenk 1975; Mitchell & Kawakatsu 1973a, b; Sluys 1992; Sluys et al. 2005).
Notes on ecology and distribution. Both flatworm species were sampled in a single set of pools in travertine rock ( Figs. 3, 4 View FIGURES 1–4 ) situated in the eastern branch of “Areias de Cima” cave, thus being syntopic. The water hydrochemistry at the date of collection was as follows: pH = 8.0, temperature = 17.9°C, conductivity = 117.7 and dissolved oxygen = 5.30 g/l. The small set of pools ( Fig. 4 View FIGURES 1–4 ) is formed by water percolating from the epicarstic zone. Considering the fact that during flooding some pools can be submerged by the stream, the species may have colonized these pools from the stream. However, no specimens of either species were found in the streams during four sampling events in the cave streams between 2012 and 2014, in both rainy and dry seasons, in a total of 13 areas inside the caves. This fact strongly suggests that the main habitat of both species is the epikarst, and not the lothic subterranean system. Immediately adjacent to this sampling site, the troglobitic amphipod Hyallela epikastica Rodrigues et al., 2014 was discovered. This crustacean species was also found only at this site, thus also suggesting an epikarstic origin ( Rodrigues et al. 2014).
Usually, species presenting similar habits (especially feeding habits or strategies) tend to exclude each other when co-occurring in a given habitat ( Hardin, 1960). Considering the restricted food supply typically observed in caves, competitive exclusion even may be stronger. Thus, the occurrence of two sympatric flatworm species living in such a restricted and specialized habitat would be possible only if competitive exclusion is weak. The body size of both species is quite different, with G. arenicola being almost 3 times longer than G. paucipunctata , thus suggesting that the species may be exploring different microhabitats or even distinct resources in the same habitat. In another karst area in Brazil, distinct species of amphibious Styloniscidae isopods were also observed sharing the same set of pools in travertine rock (R. Ferreira, unpublished data). Similar differences in size were observed in the isopod species, suggesting that size differentiation could act indeed to diminish competitive exclusion in such restricted habitats.
The morphological features of both species, which show small eyes and an unpigmented or only weakly pigmented body, as well as their location in pools formed by percolating water suggest that they are stygobiont. The fact that both species were collected only once at a single site in the period between July, 2012 and February, 2014, when four expeditions were made into this cave system, suggests that they have a very restricted distribution. The “Areias system” is a well-studied cave system, being considered as the richest system for subterranean fauna in Brazil ( Trajano 2007; De Ázara & Ferreira 2013; Rodrigues et al. 2014) and also one of the two hotspots of subterranean biodiversity in South America ( Souza-Silva & Ferreira 2016); it is located in a preserved area (Parque Estadual Turístico do Alto Ribeira). Hence, the environment from where both flatworms were collected seems to be well protected.
| MZUSP |
Museu de Zoologia da Universidade de Sao Paulo |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Girardia paucipunctata Hellmann & Leal-Zanchet
| Hellmann, Lindsey, Leal-Zanchet, Ana Maria & Ferreira, Rodrigo Lopes 2018 |
Hyallela epikastica
| Rodrigues et al. 2014 |
G. capacivasa
| Sluys & Kawakatsu 2005 |
G. capacivasa
| Sluys & Kawakatsu 2005 |
G. avertiginis
| Sluys 2005 |
G. avertiginis
| Sluys 2005 |