Eulimnogammarus messerschmidtii Bedulina et Tachteew
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3838.5.2 |
|
publication LSID |
lsid:zoobank.org:pub:33D621CE-0856-42F6-BEA9-A75A458ABCE4 |
|
DOI |
https://doi.org/10.5281/zenodo.6140228 |
|
persistent identifier |
https://treatment.plazi.org/id/BC2587EA-E073-5D7B-2BFF-BE549E599309 |
|
treatment provided by |
Plazi |
|
scientific name |
Eulimnogammarus messerschmidtii Bedulina et Tachteew |
| status |
sp. nov. |
Eulimnogammarus messerschmidtii Bedulina et Tachteew View in CoL , sp. n.
Figures 8–15 View FIGURE 8
Eulimnogammarus cyanoides View in CoL (part.). Bazikalova 1945: 209 –210.
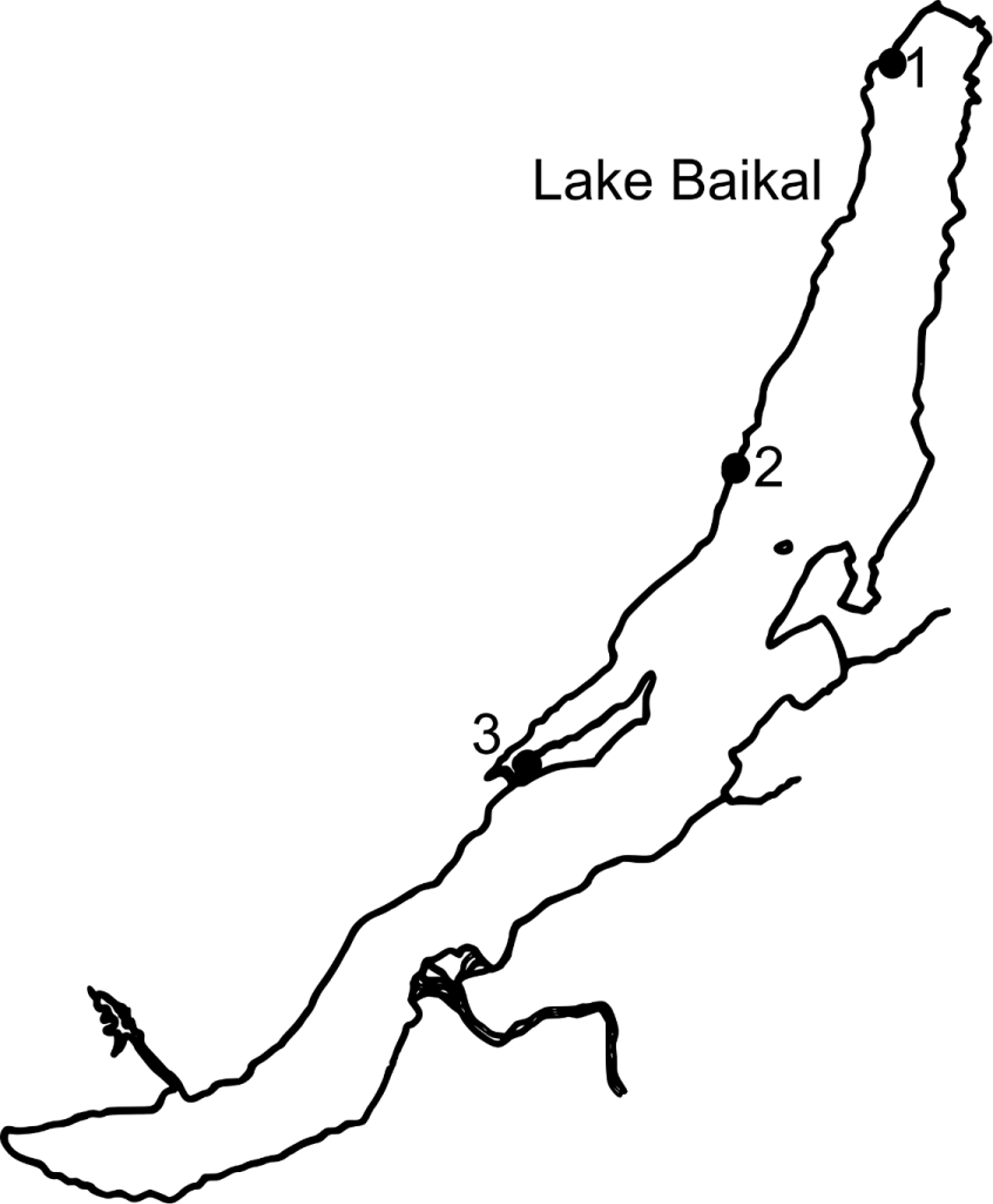
Type specimens. Holotype: male, 16.0 mm, “red” morph., ISU 12/1114, 21.07.2012, Russia, Lake Baikal, northern part near the delta of the Tyya River, the temporal island in the shoal, at a depth of 30−50 cm, pebbles and sand, macrophytes, sampling using a hand-net, temperature 15−20°C, pH 7.5 (Leg. D.S. Bedulina, T. Luckenbach). Type locality: coordinates, 55°37’5.24”N, 109°21’14.45”E (see Fig. 1 View FIGURE 1 , point 1).
Paratypes: female, 15.0 mm, “red” morph., ZIN 1/88513, same sampling locality as the holotype; 2 females (15.2 and 17.5 mm), “red” morph., ISU 12/1115, same sampling locality as the holotype; male, 18.0 mm, “blue” morph., ISU 12/1116, same sampling locality as the holotype; 2 females (15.2 and 13.8 mm), “blue” morph., LIN 1131, same sampling locality as the holotype; 2 males (12.0 and 15.6 mm), 4 females (12.4, 12.5, 12.7 and 12.8 mm), ISU 12/1117, 25.06.2006, Russia, Baikal Lake, Cape Bol’shoy Solontsovy, southern part, 54°10’23” N, 108°21’01” E (see Fig. 1 View FIGURE 1 , point 2), water edge, stones, temperature 5.5°C, sampling using a hand net (Leg. V.V. Takhteev, D.S. Bedulina); male, 12.5 mm, 3 females (12.7, 13.0 and 13.9 mm), ZMB 28096, same sample site; 2 males (13.0 and 14.3 mm), 2 females (12.3 and 13.4 mm), LIN 1132, same sampling site; male, 13.8 mm, 3 females (12.1, 13.3 and 13.3 mm), ZMK 246 (Inventory book of the stock collection of invertebrates, without insects) 96 (database of type materials of invertebrates, without insects), same sample site; 2 males (8.7 and 12.8 mm), female, 15.2 mm, ISU 12/1118, 22.06.2002, Russia, Baikal Lake, Olkhon Island, Kharin-Irgi Bay, 53°04’10” N, 106°55’52” E (see Fig. 1 View FIGURE 1 , point 3), water edge (at a depth of 0−30 cm), sampling using a hand net, stones, temperature 7.5°C (Leg. E.B. Govorukhina, V.V. Takhteev); male, 11.4 mm, 3 females (11.0, 11.4 and 13.3 mm), ZIN 2/88514, same sample site; 2 males (9.2 and 10.7 mm), 2 females (13.2 and 13.5 mm), ZMH K 43660 View Materials , same sample site; male, 11.7 mm, female, 10.1 mm, MZH 125.886, same sample site.
Other material examined. For the population analysis, an additional sample of 165 specimens collected from the sampling point 3 (Olkhon, Kharin-Irgi Bay, same date of collection) was used. Additionally, a pool of all paratypes (18 specimens) from the sampling point 3 (Cape Bol’shoy Solontsovy) was examined.
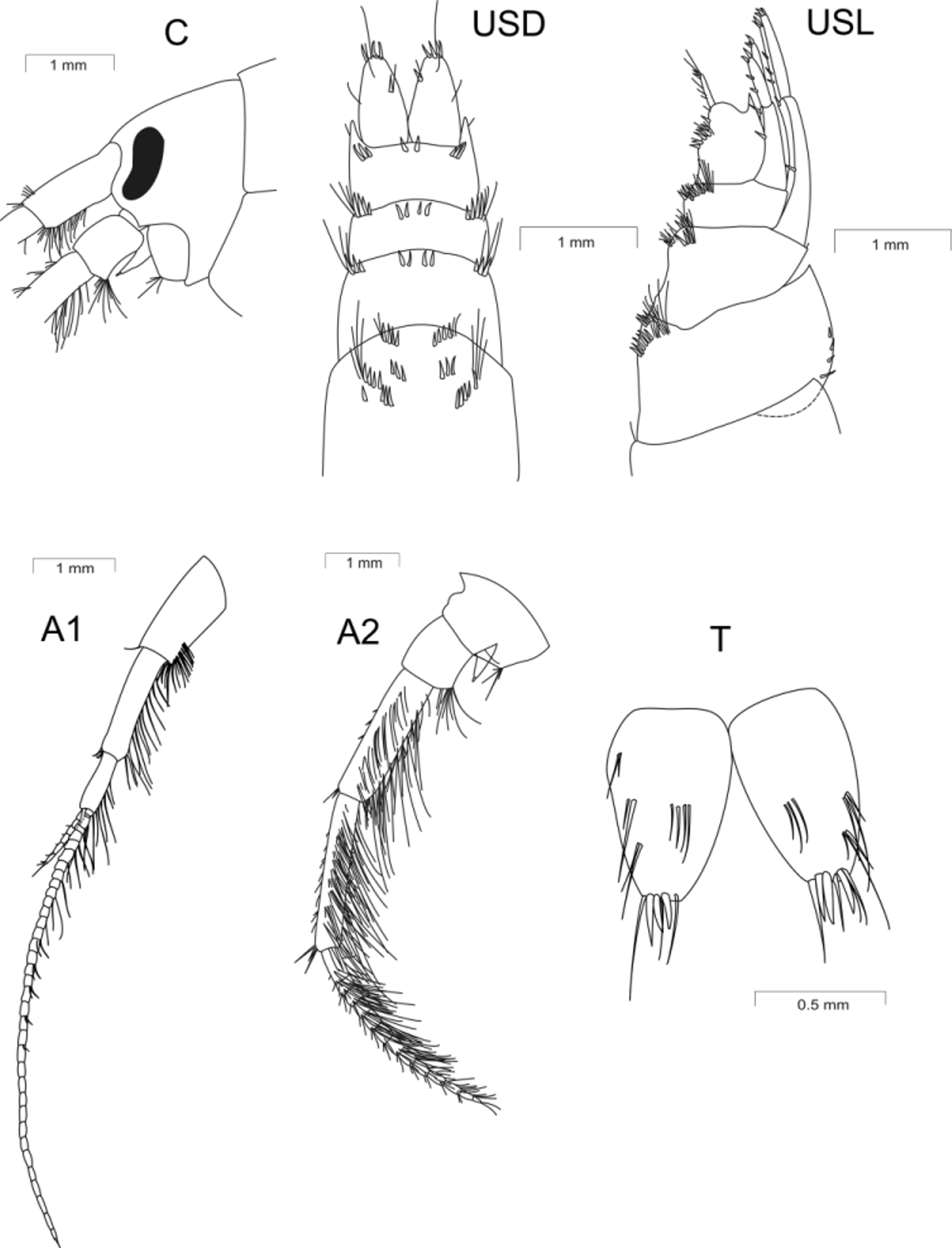
Diagnosis. All characteristics should be considered together for species identification. Spines only on four last body segments, being combined with setae. Eyes narrow and curved. Flagella of antennae 1 and 2 with bunches of long, dense setae. Basal article of peduncle of antenna 1 without thorns on inferior-anterior angle. Antenna 1 not exceeding half of body length. Basis of pereopod 7 with poorly developed rectangular lobe on its inferior-posterior margin. Uropod 3 with dense simple setae, without plumose setae; outer ramus with small second article. Telson lobes bearing only apical spines and no lateral spines. Body length of adult specimens ranging from 7.5 to 18.0 mm in males and from 8.0 to 17.5 mm in females.
Description. Male: Body smooth and Gammarus -like: compact, lateral narrowed. Pleon. Segment 1 bare, and segment 2 bearing only one pair of setae on posterior margin. Segment 3 with 3−4 paired rows of spines on dorsal surface, and sparse setae, quite long in two of the groups and sometimes protruding to the middle of next segment.
Urosome. All three segments bearing two paired groups of spines (two median and two lateral); first and second segments with sparse, but quite long setae not protruding past the posterior margin of following segment.
Head. Head slightly convex dorsally, with very short rostrum. Interantennal lobes with indistinct notch. Eyes black, narrow and curved (narrow-reniform), and their vertical diameter exceeding horizontal diameter by almost 3-fold.
Antenna 1 slightly shorter than 1/2 of body length and slightly longer than antenna 2. Basal peduncular article slightly shorter than head and without spines on its inferior-anterior margin. Distal half of inferior margin bearing dense setae, elongated at the end of article. Peduncle article 2 slightly longer than basal, whereas article 3 is 0,7 times as long as article 2. Both bearing rows of bunches of long setae on their inferior margin. Primary flagellum with 20−36 short articles; accessory flagellum with 3−5 and in rare cases, 7 articles.
Antenna 2. Article 1 swollen, with a bunch of setae; antennal cone markedly shorter than third article of the peduncle; article 3 bearing a bunch of setae of varying lengths on its inferior-anterior angle. Article 4 slightly shorter than article 5, and both (in addition to the flagellum) bearing bunches of long, dense setae on their inferior margin. Flagella comprise up to 10−13 articles; calceoli absent.
Mandible. Incisor with 5 long teeth. Dental row with 8 setae. Palp elongated; article 1 bearing a group of setae; article 2 narrow, with long, dense setae (D2-setae, after Lowry & Stoddart 1993) on its distal half. Terminal article lanceolate, with numerous A3- and E3-setae; D3-setae bunch 2/3 of article length; setae equal and gradually decreasing in length towards distal end.
Maxilla 1. Outer plate containing 11 oblique-pectinate spine-teeth. Inner plate wide, bearing 17−18 plumose setae. Palp slightly narrower and longer than outer plate (together with spines). Maxilla 2. Outer plate slightly longer than inner plate, and both plates with dense setae on their distal ends. Inner plate bearing 17 plumose setae in oblique row.
Maxilliped. Outer plate exceeding 2/3 of middle article of palp, and distal end widening and markedly wider than inner plate. Distal end of inner plate narrowing, trapeziform and bearing 3 large tapering teeth and several small, indistinct teeth at its distal end. Palp with dense groups of setae on its middle and terminal articles; middle article quite wide, whereas terminal article narrower than middle; dactylus 2/3 of length of terminal article.
Coxal plates with smooth rounded anterior angles, bearing single short setae on margins and on outer side. Coxal plate 1 shorter than coxal plates 2−4. Coxal plates 1−3 linguiform, and coxa 4 with obtuse angle on inferior 1/3 of posterior margin.
Gnathopod 1. Propodus narrow and amygdaline, shorter or equal to head length and 1/3 times as long as propodus of gnathopod 2; palmar margin with weak notch, gradually aligning to inferior. Inferior margin bearing 6 bunches of setae, all of which, with exception of first bunch, combined with spines increasing in length from proximal to distal. Dactylus curved markedly.
Gnathopod 2. Propodus with parallel superior and inferior margins, and palmar margin delimited inferiorly by an angle; inferior margin bearing numerous bunches of dense setae.
Pereopods 3 and 4 almost equal in length, and their bases curved. Carpi and propodi almost equal in length and slightly shorter than meri. Articles with bunches of spines and setae; longest setae on posterior margins of meri and carpi. Dactyli short and thick, and their tip curved markedly.
Pereopods 5−7. Pereopod 6 almost a third longer than pereopod 5 and slightly shorter than pereopod 7. Basis of pereopod 5 wide (its length exceeding width by 1/4), posterior margin almost straight, and its inferior portion forming rather wide and slightly declining lobe. Basis of pereopod 6 tapering distally; posterior margin slightly excavated and with small rectangular lobe on the inferior margin. Basis of pereopod 7 twice as long as wide; posterior margin tapering in the inferior one-third and with more indistinct rectangular lobe.
Posterior margins of bases 5−7 with slightly elongated setae (thus, this species’ morphology is different from the majority of other Eulimnogammarus ).
Meri and carpi equal in length, and propodi slightly longer, all bearing groups of spines and setae. Dactyli short and thick, with strongly curved tips.
Epimeral plates of first pleonal segment small; on segment 2 with dulled inferior-posterior angle; on segment 3 wide and with a row of small spines along their inferior margins. Inferior-posterior angle of epimeral plates almost straight or slightly sharp or obtuse.
Uropods 1 and 2 armed only with spines. Peduncles in both pairs of equal length, and rami slightly shorter than peduncles. Spines present at both ends and sides of rami. In uropod 1, rami long (longer than apexes of uropod 2 rami); outer ramus slightly longer than inner ramus. Outer ramus of uropod 2 slightly shorter than inner ramus.
Uropod 3 is 1/5−1/6 the length of body; outer ramus>3-fold longer than peduncle and 4.0−4.5-fold longer than inner ramus. Rudimentary article 2 present on outer ramus. Both rami with groups of spines and dense, simple setae; plumose setae absent.
Telson notched up to the base; lobes tapering, and each with 3 apical spines and groups of setae on apexes and dorsal surface; setae not exceeding length of lobes; exterior and interior margins without spines. Body length up to 18 mm.
Female. Propodus of gnathopod 1 is 0.7 times as long as head, its superior and inferior margins parallel, and palmar margin markedly skewed, but delimited with inferior margin and not concave; marginal spines large; inferior margin with fewer spines than in males.
Propodus of gnatopod 2 slightly shorter and narrower than in gnatopod 1 and almost rectangular; superior and inferior margins parallel, and palmar and inferior margins forming almost right angle.
Furthermore, there is a distinct difference between the right and left maxillae 1 of the paratype (female). Right maxilla 1 with considerably expanded apical article of palp, exceeding width of outer plate and bearing 6 tapering small teeth and sparse short setae on its distal margin.
Lower lip lobes wide oval, with thin setules along inferior margins.
Body length up to 17.5 mm. Sexual differences in body length are insignificant.
Intravital color. Two sympatric morphs of the species were identified that clearly differ based on color but are highly similar after fixation. The first morph is blue-grey with antennae that have orange stripes ( Fig 9 A). This morph is similar in color to another widespread species in the Baikal littoral zone— Eulimnogammarus cyaneus (Dybowsky, 1874) (see “Comparative remarks”). The second morph is ginger-red (light-orange) and has antennae with orange stripes that are darker than the stripes above; the urosomital segments carry a dark-red spot ( Fig. 9 B).
Color polymorphism has been described previously for other Baikal species: E. cyaneus , E. vittatus (Dybowsky, 1874) and Parapallasea puzyllii nigra (Garjajew, 1901) ( Takhteev 1993; 2000). The carotenoid composition may affect the color ( Goodwin, 1960; Wade et al., 2012).
Variation. The length of the flagella of antennae 1 and 2 and uropod 3 may vary a bit. The notch on the anterior margin of the eye may be less indistinct or distinct. The setae on the posterior margin of basis pereopod 7 may be of varying length; however, in general, they are longer than normal for Eulimnogammarus species. The epimeral plates 2 and 3 may bear sparse, medial-length setae on their inferior and posterior margins; small spines on the third pair may be unapparent. Spines on the last body segments vary in size. The spine and setae armaments on the left and right sides of these segments may show fluctuating asymmetry (see Fig. 10 View FIGURE 10 ). The spines on the third pleonal segment form 2−4 rows.
Comparative remarks. Because of intravital color (“blue” morph) of this species it may be confused with E. cyaneus (Dybowsky) and also this species may be inaccurately identified as E. cyanoides using the common key of Bazikalova (1945), differences between these species are stated below.
The most considerable differences between E. messerschmidtii sp. n. and E. cyanoides : 1. Spines are present on the 4 last and not on the 3 last body segments; setae on the urosomital segments do not exceed the length of the following segment. 2. Eyes are narrow and curved. 3. The basal article of the peduncle of antennae 1 without spines, but with dense setae on the distal half. 4. The propodus of gnathopod 1 is considerably smaller and does not exceed the length of the head.
Differences between E. messerschmidtii sp. n. and E. cyaneus : 1. The body size is larger ( E. cyaneus —up to 11−13 mm and rarely up to 15 mm). 2. Spines are present on the third pleonal segment (in E. cyaneus it has only dense setae). 3. The terminal article of the palp of the mandible bears ordinary brush (D3-setae). Setae do not differ significantly in length and are “clipped” equally (in E. cyaneus , the brush shows long unequal setae). 4. The posterior margin of the basis of pereopod 7 in E. cyaneus is distinct in its distal part and is elongated to a sharpened angle, whereas in E. messerschmidtii sp. n., the inferior portion of the posterior margin shows an indistinct rectangular lobe only. 5. The outer ramus of uropod 3 of E. messerschmidtii sp. n. is 4.0−4.5-fold longer than the inner ramus, and its second article is rudimentary; the outer ramus of E. cyaneus is only 3-fold longer than the inner ramus, and the second article is distinct.
Etymology. This species is named in honour of the German scientist-encyclopedist Daniel Gottlieb Messerschmidt (1685–1735) who was one of the first members of the Russian (St. Petersburg) Academy of Sciences. D.G. Messerschmidt accomplished the first scientific expedition to Siberia (1720–1727). He was the first researcher of Lake Baikal who described the nature resources (including numerous plant species) and performed ethnologic studies of local tribes and peoples in regions from the Lake Baikal area to Eastern Siberia, which are described in his 4-volume work “Forschungsreise durch Sibirien (1720–1727)”.
Distribution, ecology and biology of populations. To date, occurrence of E. messerschmidtii sp. n. is documented for the 3 sampling points stated above (see “ Type specimens”; Fig. 1 View FIGURE 1 ). Based on the descriptions of the sites of sampling, the species is assumed to inhabit the cold open shore and the shallow waters of Lake Baikal, the latter of which can warm up to 20°C or more during summer (Kharin-Irgi Bay, shallow water of the Tyya River). The dwelling substrata include boulders and pebbles with underlying sand. Apparently, E. messerschmidtii sp. n. is one of the typical, and even mass, species of the littoral zone of Lake Baikal; however, its distribution is limited by the northern half of the lake and the Maloe More strait (including its south tip—Olkhonskie Vorota).
E. messerschmidtii may perform horizontal migrations because it is found to be either highly abundant or absent at the same sites; i.e., it was found en masse in Kharin-Irgi Bay (Olkhon island) on 22.06.2002, but no specimens were found at the same site on 24.06.2006.
Fig. 16 View FIGURE 16 demonstrates the sex-age structure of the population of E. messerschmidtii sp. n. in Kharin-Irgi Bay (point 3) on 22.06.2002. Males comprised 33.7% of the population, and the male:female ratio was 1:2. Because the majority of the females—77.1%—were carrying eggs (and some had released their eggs already), it can be assumed that the period of reproduction is not very extended and that it began in May or even at the end of April. This period of reproduction is typical for another highly abundant upper-shore species— E. cyaneus ( Bazikalova 1941; Weinberg et al. 2002; Govorukhina 2005).
The small sample of E. messerschmidtii sp. n. from the Bolshoy Solontsovy cape, collected at the same time of year (25.06.2006), comprised 1 of 12 females in the 4th stage and 11 in the 5th stage of the life cycle (see: methods). Type specimens from the shallow water of the Tyya River delta (21.07.2012) included only one female in the 5th stage and 4 females in the 2nd stage. They were large, had apparently passed out of reproduction and had already molted.
Despite the limited amount of available material, the second part of July may be assumed as the end of reproduction cycle.
Based on reproduction time three main groups of amphipods from Lake Baikal are distinguished ( Bazikalova 1941; Gavrilov 1949). The first group includes species that reproduce from spring prior to ice breakage until the summer months; species of the second group copulate during autumn and carry eggs until spring; reproduction of species from the third group is observed during most of the year or year-round.
Apparently, E. messerschmidtii sp. n. belongs to the first group of species with a spring-summer reproduction period, which may be initiated by the increase in the photoperiod to 12 hours or more. Temperature cannot play a significant role because in the middle of May, the temperature in the Southern part of Lake Baikal does not exceed 2.5°C ( Govorukhina 2006) and solid shore ice remains in the Northern part.
Sex and age groups Body length, mm
7– 8– 9– 10 – 11– Males Weight, mg 6.5±0.7 7.0 13.1±0.7 16.7±0.6 21.3±1.0
n 2 1 11 20 7
Females, Weight, mg – 10.4±0.4 12.2±0.5 15.3±0.5 18.0 2 stage n – 5 11 3 1 Females, Weight, mg – 10.0 13.3±0.4 17.3±0.3 23.2±0.6 3 stage Number of eggs – 6.0 10.4±0.7 12.6±0.4 19.0±0.9
n – 1 17 37 14 Sex and age groups Body length,mm
12– 13– 14– 15 – 16– 17– 18 Males Weight, mg 26.8±1.3 31.7±0.8 37.0 – – 53.0 n 8 3 1 – – 1 Females, Weight, mg – – – – – – 2 stage n – – – – – – Females, Weight, mg 29.7±0.7 – 48.1±1.9 51.3±5.5 – – 3 stage Number of eggs 28.3±1.0 – 43.8±4.7 39.7±9.0 – – n 10 – 6 3 – –
The mean size of all males was 10.7± 0.2 mm (n=54), whereas females were 10.4± 0.2 mm (n=111). The mean weight of all males was 19.4± 1.1 mg (n=54), whereas females weighed 20.0±1.0 mg (n=111). There were no statistically significant intersexual differences in either size or weight.
To further describe the population structure of E. messerschmidtii sp. n., which may aid in calculating growth rates and life cycle in further studies, the mean size and weight of females at different stages was measured.
The mean size of females in the 1st stage was 7.7± 0.6 mm, and the mean weight was 7.0±1.0 mg (n=3); females in the 2nd stage were 9.2± 0.2 mm and 12.6± 0.6 mg (n=20); females in the 3rd stage were 10.8± 0.2 mm and 22.1± 1.1 mg (n=88).
As shown in table 1, there are significant differences in female length and weight between the 2nd and 3rd group. As mentioned previously, the life cycle of Baikal amphipods is generally quite long, i.e. the life cycle of the mass species Eulimnogammarus of the littoral zone is 3 to 5 years ( Govorukhina 2005). It is possible that 2nd-stage E. messerschmidtii sp. n. females that were collected in June belong to the previous year’s generation and that females in the 3rd stage (participated in reproduction) are older and are aged almost two years.
The mean index of the absolute one-time fertility is 18.0±1.2 eggs (n=88), and the index fluctuates from 5 to 57 eggs, depending on size. The increase in fertility correlates exponentially with increasing body size and almost proportionally to increasing body mass ( Table 1). The relative one-time fertility was 12.8%.
The small sample from point 2 (Cape Bolshoy Solontsovy) included specimens of the following body lengths: males, 12.0− 15.6 mm, mean 13.5± 1.3 mm (n=6); females at 3rd −5th stage, 12.3 to 13.9 mm, mean 12.9± 0.5 mm (n=12).
Specimens from the type sampling point (Tyya River delta) were larger: males, 16.0−18.0 mm; females, 2nd stage, 13.8−17.5 mm, a female in the 5th stage, 15.2 mm. Evidently, only older specimens were collected from points 1 and 2.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Eulimnogammarus messerschmidtii Bedulina et Tachteew
| Bedulina, Daria S., Ta, V., Pogrebnyak, Svyatoslav G., Govorukhina, Ekaterina B., Madyarova, Ekaterina V., Lubyaga, Yulia A., Vereshchagina, Kseniya P., Timofeyev, Maxim A. & Luckenbach, Till 2014 |
Eulimnogammarus cyanoides
| Bazikalova 1945: 209 |