Clinodiplosis comitis, Dorchin & Joy & Hilke & Wise & Abrahamson, 2015
|
publication ID |
https://doi.org/ 10.1111/zoj.12234 |
|
persistent identifier |
https://treatment.plazi.org/id/81198784-FF90-FFFE-9123-8EB7FFA6FD85 |
|
treatment provided by |
Felipe |
|
scientific name |
Clinodiplosis comitis |
| status |
SP. NOV. |
CLINODIPLOSIS COMITIS DORCHIN SP. NOV.
Hosts and biology
This species lives as an inquiline in Asphondylia monacha and A. pseudorosa sp. nov. galls. Dozens of tiny, yellowish larvae were found frequently among the rosette leaves ( Fig. 21 View Figures 15–22 ), but we never observed them actually interacting with larvae of the gall inducer. They leave the gall to pupate in the ground and adults emerge 10–14 days later. This is an extremely delicate cecidomyiid whose dark eyes stand out on the background of an otherwise whitish body. Several consecutive generations appear to develop during spring and summer, and larvae of the last generation most probably overwinter in the ground.
Adult
Tiny whitish-hyaline gall midge with black eyes.
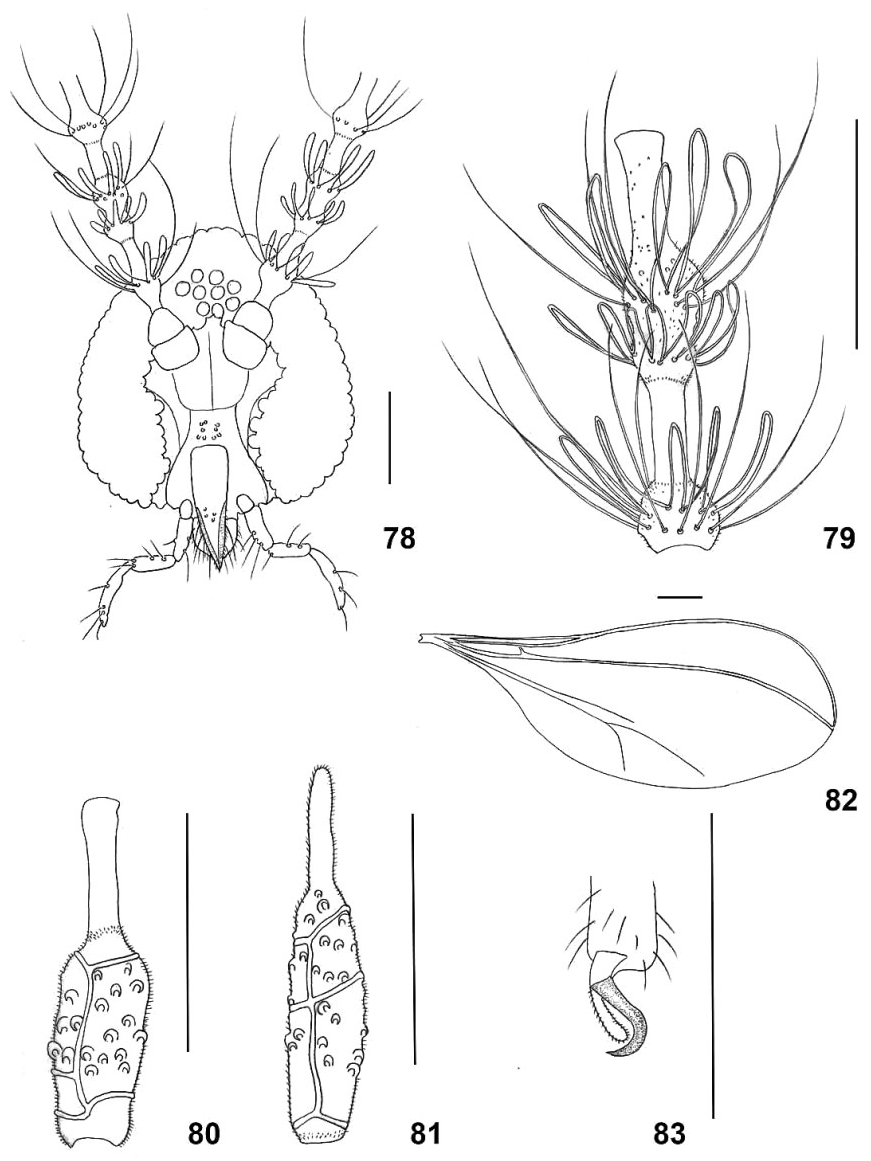
Head ( Fig. 78 View Figures 78–83 ): Small dorsal projection at top of vertex present just behind eyes, bearing two setae. Eye facets round. Palpus four-segmented; segment 1 about as long as wide, segments 2 and 3 about 2.5 times as long as segment 1, segment 4 about 4.0 times as long as segment 1; all segments with fine, long setae and otherwise covered by microtrichia. Face with four or five short setae on each side. Labrum and labella pointed, strongly setose. Antenna: 12 flagellomeres in both sexes; first two flagellomeres fused. Male flagellomeres 1– 11 trinodal ( Fig. 79 View Figures 78–83 ): first node setulose, with basal whorl of strong setae, distal whorl of long-looped circumfila, followed by long, bare neck; second node setulose, with one median circumfilar whorl, followed by third setulose node, with basal whorl of strong setae and whorl of long-looped circumfila, followed by long, mostly bare neck. Flagellomere 12 with third node followed by long and thin vestigial, setulose appendage, 0.2–0.3 times as long as flagellomere. Both proximal and distal necks longer in distal flagellomeres than in proximal flagellomeres: neck 1 to node 1 ratio 0.83–1.37 for flagellomere 3 (N = 8), 1.10–1.47 for flagellomere 7 (N = 7); neck 2 to nodes 2 + 3 ratio 0.66–0.90 for flagellomere 3 (N = 8), 0.89–1.14 for flagellomere 7 (N = 7). Female flagellomeres cylindrical, setose and setulose, with simple circumfila and long bare necks of same relative length throughout antenna ( Fig. 80 View Figures 78–83 ); neck to node ratio for flagellomere 7, 0.61–0.80 (N = 12). Flagellomere 12 with long vestigial appendage, about 0.3 times as long as flagellomere, setose and setulose, rounded apically ( Fig. 81 View Figures 78–83 ).
Thorax: Legs: tarsal claws bent beyond mid-length, untoothed ( Fig. 83 View Figures 78–83 ); empodia almost reaching bend in claw. Wing ( Fig. 82 View Figures 78–83 ): completely transparent, with sparse, delicate hairs; length 1.60–2.00 mm in male (N = 11), 1.40–2.20 mm in female (N = 10). R1 joins C at third of wing length, R5 joins C far beyond wing apex, Rs incomplete, situated around midlength of R1; M weak, CuA forked, with CuA2 strongly curved posteriad.
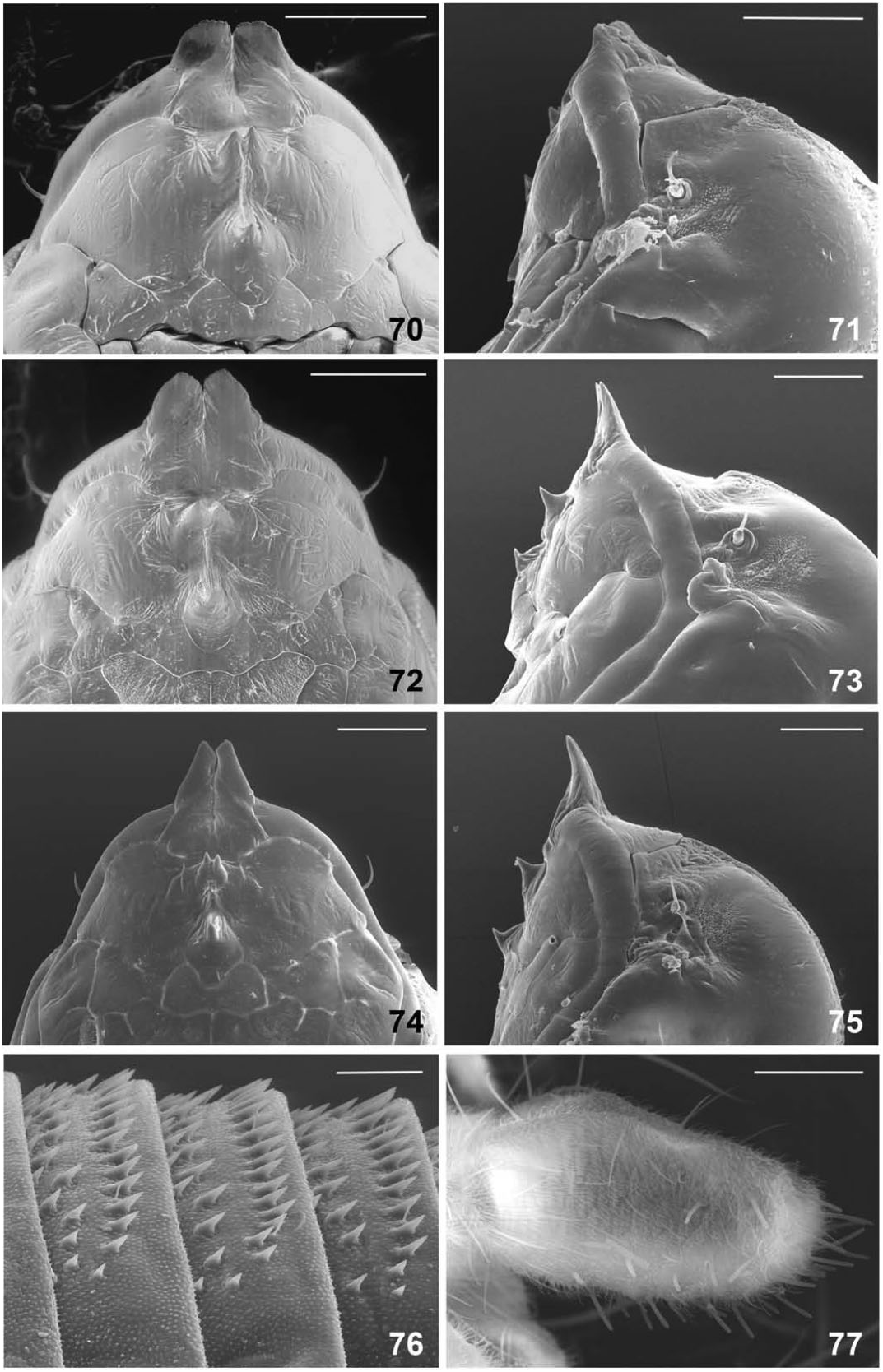
Female abdomen ( Fig. 84 View Figures 84–87 ): Sclerites rectangular, virtually undifferentiated from surrounding membrane; tergites with posterior row and one or two median rows of setae; sternites with uniformly scattered setae; no discernible trichoid sensilla. Ovipositor protractible, about 3.5 as long as sternite 7. Cerci large, setose and setulose, ventral and apical areas with numerous thicker, blunt sensory setae ending in apical pore ( Fig. 77 View Figures 70–77 ).
Male abdomen: Segments and setation as described in female.
Male terminalia ( Fig. 86 View Figures 84–87 ): Gonocoxites slender, with strong setae on mid-distal half. Gonostylus long and slender, only slightly arched, about same width throughout length, with numerous evenly spread short setae, bearing small apical tooth. Aedeagus wide, conical, extending far beyond hypoproct, with two pairs of pits on apical third; slightly constricted at level of apical pits towards rounded apex. Hypoproct widest distally, with wide, deeply concave notch apically, evenly setulose dorsally except for bare, widened sections along apicolateral margins. Cerci trapezoid, separated almost to base by a deep notch, narrowing abruptly at half-length to form triangular lobes; evenly setulose, with several long setae apically and one strong median seta at base of triangular lobe.
Pupa
Unknown.
Larva (third instar) ( Figs 85, 87 View Figures 84–87 )
Pale yellow, slender. Length: 1.36–2.19 mm (N = 12). Integument covered by shallow, acute bumps. Antennae about twice as long as wide; cephalic apodeme as long as head capsule. Spatula with two triangular teeth and long, slender shaft, on each side with two groups of three tiny lateral papillae with no perceptible setae ( Fig. 85 View Figures 84–87 ). Terminal segment with one large and two smaller pairs of coniform papillae, and fourth pair bearing long setae ( Fig. 87 View Figures 84–87 ).
Diagnosis
This species is unique among all 45 described species of Clinodiplosis in North America for the shape and setation of the male hypoproct and the large female cerci with their ventral group of blunt sensory setae.
Etymology
The species name is derived from the Latin word for ‘companion’, with reference to the fact that it accompanies Asphondylia galls without causing apparent damage to the gall inducer.
Notes
Clinodiplosis View in CoL is a large cosmopolitan genus represent- ed in North America by 45 described species and many undescribed species ( Gagné, 1994; Gagné & Jaschhof, 2014). Whereas most European species appear to be mycophagous, many New World species are inquilines, gall inducers, or even predators ( Gagné & Jaschhof, 2014), but the life history of many is unknown because they were caught in flight. Morphological attributes of the male genitalia, and in particular of the cerci, are the best taxonomic characters in the genus, although in some cases there may be considerable intraspecific variability that renders these characters unusable ( Skuhravá, 1973). The shape and setation of the male hypoproct and the large female cerci, with their blunt sensory setae, in C. comitis make this species unique among all described North American species (R. Gagné, pers. comm.). At present we do not know how specific its association with Asphondylia View in CoL galls on goldenrods is, but we never reared it from galls of other cecidomyiids on these plants.
Type material
Holotype: ♂, USA, PA, Millersburg, 29 June 2007, N. Dorchin and M.J. Wise, ex Asphondylia pseudorosa sp. nov. bud gall on Euthamia graminifolia (TAUI) .
Paratypes: 8 larvae, USA, PA, Lewisburg, 8 August 2005, N. Dorchin, ex Asphondylia pseudorosa sp. nov. bud galls on Euthamia graminifolia (4 USNM, 4 TAUI); 7 larvae, USA, PA, Rt. 487 (41°21.2′N, 76°17.8′W), 31 July 2006, N. Dorchin, ex Asphondylia monacha bud galls on Solidago juncea ; 3♂, 6♀, same data as holotype (1♂ & 1♀ USNM, others TAUI); 4♂, 3♀, USA, PA, White Deer Creek, 4 July 2007, N. Dorchin and D. Ryan, ex Asphondylia pseudorosa sp. nov. bud galls on Euthamia graminifolia (TAUI) .
Other material examined
2♂, 2♀, USA, PA, Millersburg , 29 June 2007, N. Dorchin & M.J. Wise ; 4♂, 3♀, USA, PA, White Deer Creek , 4 July 2007, N. Dorchin & D. Ryan .
| PA |
Universidade Federal do Oeste do Pará |
| USNM |
Smithsonian Institution, National Museum of Natural History |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Clinodiplosis comitis
| Dorchin, Netta, Joy, Jeffrey B., Hilke, Lukas K., Wise, Michael J. & Abrahamson, Warren G. 2015 |
C. comitis
| Dorchin & Joy & Hilke & Wise & Abrahamson 2015 |