Cicadetta anapaistica subsp. lucana, 2015
|
publication ID |
https://doi.org/ 10.1111/zoj.12212 |
|
publication LSID |
lsid:zoobank.org:pub:4909845B-F89E-4F06-9E6D-02DADBE44AD9 |
|
DOI |
https://doi.org/10.5281/zenodo.5413649 |
|
persistent identifier |
https://treatment.plazi.org/id/E6884153-C0E4-4B1C-A516-A295E05142E9 |
|
taxon LSID |
lsid:zoobank.org:act:E6884153-C0E4-4B1C-A516-A295E05142E9 |
|
treatment provided by |
Felipe |
|
scientific name |
Cicadetta anapaistica subsp. lucana |
| status |
subsp. nov. |
CICADETTA ANAPAISTICA LUCANA HERTACH View in CoL SSP. NOV.
Type material
The type series consists of 16 males and two females, representing southern, central, and northern populations. It is kept in the NHMB (holotype), ETHZ, NMBE and in one private collection.
Holotype male
Verbatim label information: ‘nördl. Mezzana Torre , BASI, I/ 40.0054° / 16.1681°, 1030 m asl / 18.7.2013, leg. T. Hertach / Collection Code No. 19.012’ (label rectangular, white, printed) and ‘HOLOTYPUS ♂/ Cicadetta anapaistica lucana ssp. nov. / Hertach 2014’ (label rectangular, light red with dark-red margin, printed) ( NHMB). GoogleMaps
Paratypes
All paratypes with labels ‘ PARATYPUS XX Y, Cicadetta anapaistica lucana ssp. nov. Hertach 2014’ (label rectangular, white with red margin, printed), at which ‘XX’ is the number of the paratype and ‘Y’ is the sex of the specimen. Number ‘12’ does not exist.
Paratype males, dark morph: Timpa Falascoso, Viggianello, BASI, I, 40.0184°/16.1107°, 950 m asl, 7.7.2010, leg. T. Hertach (paratype 2, coll. Hertach) ; Serra Alberigo, Viggianello, BASI, I, 40.0014°/16.1171°, 1020 m asl, 7.7.2010, leg. T. Hertach (paratype 4, coll. Hertach) ; Timpone Rotondella, Morano Calabro, CALA, I, 39.8606°/16.0953°, 980 m asl, 17.7.2013, leg. T. Hertach (paratype 6, coll. Hertach) ; Serra Alberigo, Viggianello, BASI, I, 40.0012°/16.1170°, 1040 m asl, 17.7.2013, leg. T. Hertach (paratype 7, coll. Hertach) ; Serra Alberigo, Viggianello, BASI, I, 39.9980°/16.1218°, 1070 m asl, 17.7.2013, leg. T. Hertach (paratypes 8, 9 and 10, coll. Hertach) ; N Mezzana Torre , BASI, I, 40.0054° / 16.1681°, 1030 m asl, 18.7.2013, leg. T. Hertach (paratype 13, coll. NMBE) GoogleMaps ; N Marsico Nuovo, BASI, I, 40.4659°/15.7380°, 1130 m asl, 20.7.2013, leg. T. Hertach (paratypes 14 and 15, coll. Hertach) ; N Morra de Sanctis, CAMP, I, 40.9365°/15.2410°, 820 m asl, 22.7.2013, leg. T. Hertach (paratype 17, coll. ETHZ).
Paratype males, light morph: Timpa Falascoso, Viggianello, BASI, I, 40.0184°/16.1107°, 950 m asl, 7.7.2010, leg. T. Hertach (paratype 1, coll. Hertach) ; Serra Alberigo, Viggianello, BASI, I, 40.0014°/16.1171°, 1020 m asl, 7.7.2010, leg. T. Hertach (paratype 3, coll. ETHZ) ; W Acerno, Monti Picentini , CAMP, I, 40.7545° / 15.0406°, 600 m asl, 14.7.2010, leg. T. Hertach (paratype 5, coll. NMBE) GoogleMaps ; N Morra de Sanctis, CAMP, I, 40.9365°/15.2410°, 820 m asl, 22.7.2013, leg. T. Hertach (paratype 18, coll. Hertach, putative hybrid with C. sibillae sp. nov.).
Paratype females: Serra Alberigo, Viggianello, BASI, I, 39.9980°/16.1218°, 1070 m asl, 17.7.2013, leg. T. Hertach (paratype 11, coll. ETHZ) ; N Marsico Nuovo, BASI, I, 40.4659°/15.7380°, 1130 m asl, 20.7.2013, leg. T. Hertach (paratype 16, coll. Hertach) .
MORPHOLOGY
Diagnosis
Cicadetta anapaistica lucana ssp. nov. occurs in two differently coloured morphs present in the same local populations. The dark-coloured morph resembles all other described C. montana complex species. The vast majority of C. a. lucana ssp. nov. specimens, like C. a. anapaistica , are separated from C. cerdaniensis s.str. by the predominantly dark basal junction of the anal veins (100% for C. a. lucana ssp. nov. dark morph and C. a. anapaistica , 94.4% for C. a. lucana ssp. nov. versus 5.0% for C. cerdaniensis s.str.). From C. montana s.str., many specimens are distinguished by the outer rim of costa darker than the inner rim and the radial/subcostal veins (77.8 versus 9.5%, chisquare contingency test, χ 2 = 27.9, P <0.001 for C. a. lucana ssp. nov. and 89.5% versus 9.5%, χ 2 = 51.1,
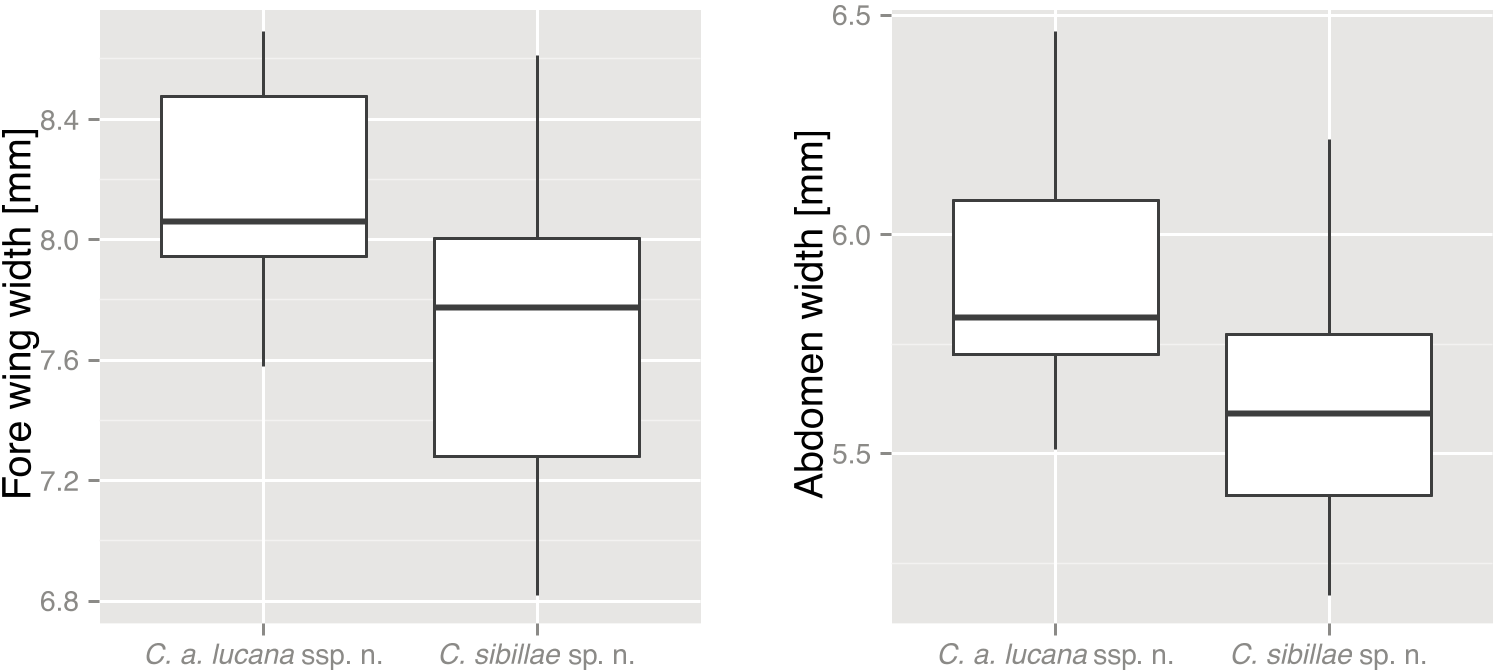
P <0.001 for C. anapaistica ). Cicadetta anapaistica lucana ssp. nov. have on average a stockier habitus in comparison with C. sibillae sp. nov., with significantly broader wings ( Fig. 5C View Figure 5 , Wilcoxon–Mann–Whitney rank sum test, W = 270, P = 0.009) and broader abdomen ( Fig. 5D View Figure 5 , Wilcoxon–Mann–Whitney rank sum test, W = 299, P = 0.002). Additionally, in C. anapaistica the pronotal collar is frontally often broader in relation to the head than in C. sibillae sp. nov., caused by a more distinct convexity ( Fig. 5B View Figure 5 , Wilcoxon–Mann–Whitney rank sum test, W = 586.5, P <0.001, for C. anapaistica versus C. sibillae sp. nov., and W = 279.5, P = 0.010, for C. a. lucana ssp. nov. versus C. sibillae sp. nov.). The lightcoloured morph is probably separated from any other described species. Closest to C. fangoana , an endemic species of Corsica, it seems to be distinguishable by the more yellowish or ochre than reddish markings on the thorax, and by the ochre coloration of the central suture and the frontal margin of the pronotum.
Description
Two colour morphs exist, with 78% dark (including holotype and females) and 22% light. Dark morphs were captured in four local populations and light ones were captured in three populations.
Measurements
Body length: 18.3 mm in holotype specimen; 17.7 ± 0.7 mm (mean ± SD) in male dark morph paratypes; 17.7 ± 1.0 mm in male light morph paratypes; 20.2 and 19.0 mm in female paratypes, respectively. Body width (abdomen, tergite 2): 6.5 mm in holotype specimen; 5.9 ± 0.2 mm in male dark morph paratypes; 5.8 ± 0.3 mm in male light morph paratypes; 6.1 and 6.2 mm in female paratypes. Forewing length: 19.9 mm in holotype specimen; 20.0 ± 0.8 mm in male dark morph paratypes; 19.4 ± 0.4 mm in male light morph paratypes; 22.5 and 20.3 mm in female paratypes. Forewing width: 8.7 mm in holotype specimen, 8.2 ± 0.4 mm in male dark morph paratypes, 8.0 ± 0.3 mm in male light morph paratypes, 9.5 and 8.8 mm in female paratypes.
Male holotype ( Fig. 4B View Figure 4 )
The holotype specimen of C. a. lucana ssp. nov. fits the detailed description of the holotype of C. a. anapaistica ( Hertach, 2011) , with the following differences. On the head, mentum dark brown (as in some paratypes of C. a. anapaistica ). On the thorax, narrow brownish band at posterior margin (as in some paratypes of C. a. anapaistica ) and lateral depressions of cruciform elevation posterior with an ochre spot, meracanthus directed caudally not laterally (as in some recently captured specimens of C. a. anapaistica ). On the abdomen, sternites and caudal margins of tergites red brown (as in some paratypes of C. a. anapaistica ). On forewings, basal median vein ochre up to the crossveins and the node (as in some paratypes of C. a. anapaistica ). Basal junction of anal veins dark or black, as in C. a. anapaistica , but not reported there. In the genitalia, basal lobe of pygofer brown.
Male paratypes of dark morph
Dark male paratypes differ from the holotype of C. a. lucana ssp. nov. and/or C. a. anapaistica (with holotype and paratypes), as follows. On the head, postclypeus rarely almost completely black. On the thorax, lateral depressions of cruciform elevation posterior normally without an ochre spot, as in C. a. anapaistica , meracanthus variable in shape and size. Rarely, lateral margin of pronotal collar frontal to the angles scarcely convex in shape and not clearly recessed (compare Fig. 5B View Figure 5 ). On forewing, colour combinations of costal and radial/subcostal veins differ sporadically, especially the exterior rim of costa and radius/ subcosta of same colour. Rarely, cubitus anterior vein and cubitus posterior vein/first anal vein almost black. Pterostigma sometimes brownish. Median and cubitus anterior vein fused on both sides for approximately 1 mm in two paratypes. Distal veins rarely with seven or nine apical cells instead of eight. In genitalia, upper lobes of pygofer rarely more angled.
Male paratypes of light morph ( Fig. 4B, G View Figure 4 )
Contrary to the dark morph several parts of the body are ochre or yellowish in colour, instead of black. On the head, postclypeus towards the frontoclypeal suture and the anteclypeus, as well as sometimes the surrounding of the compound eyes, ochre. On the pronotum, central suture, frontal margin, and pronotal collar appearing as broad ochre bands. On the mesonotum, two triangular, ochre markings central to the lateral sigilla (in one paratype mesonotum completely ochre except for the submedian and lateral sigillae, and the scutal depressions). Cruciform elevation and its lateral depressions predominantly ochre. Ventral side of thorax generally lighter (in one paratype almost completely yellowish, including opercula). On the abdomen, variable ochre fasciae at the tergites in addition to the red brown margins. Legs with light portions more dominant. One paratype with basal junction of anal veins ochre instead of dark at forewing.
Female paratypes ( Fig. 4B View Figure 4 )
Coloration does not differ from the dark morph described above, and is consequently slightly darker than the females of C. a. anapaistica . Ratio of body length to ovipositor length (including sheath) 3.1 and 2.9.
ACOUSTIC BEHAVIOUR
Diagnosis
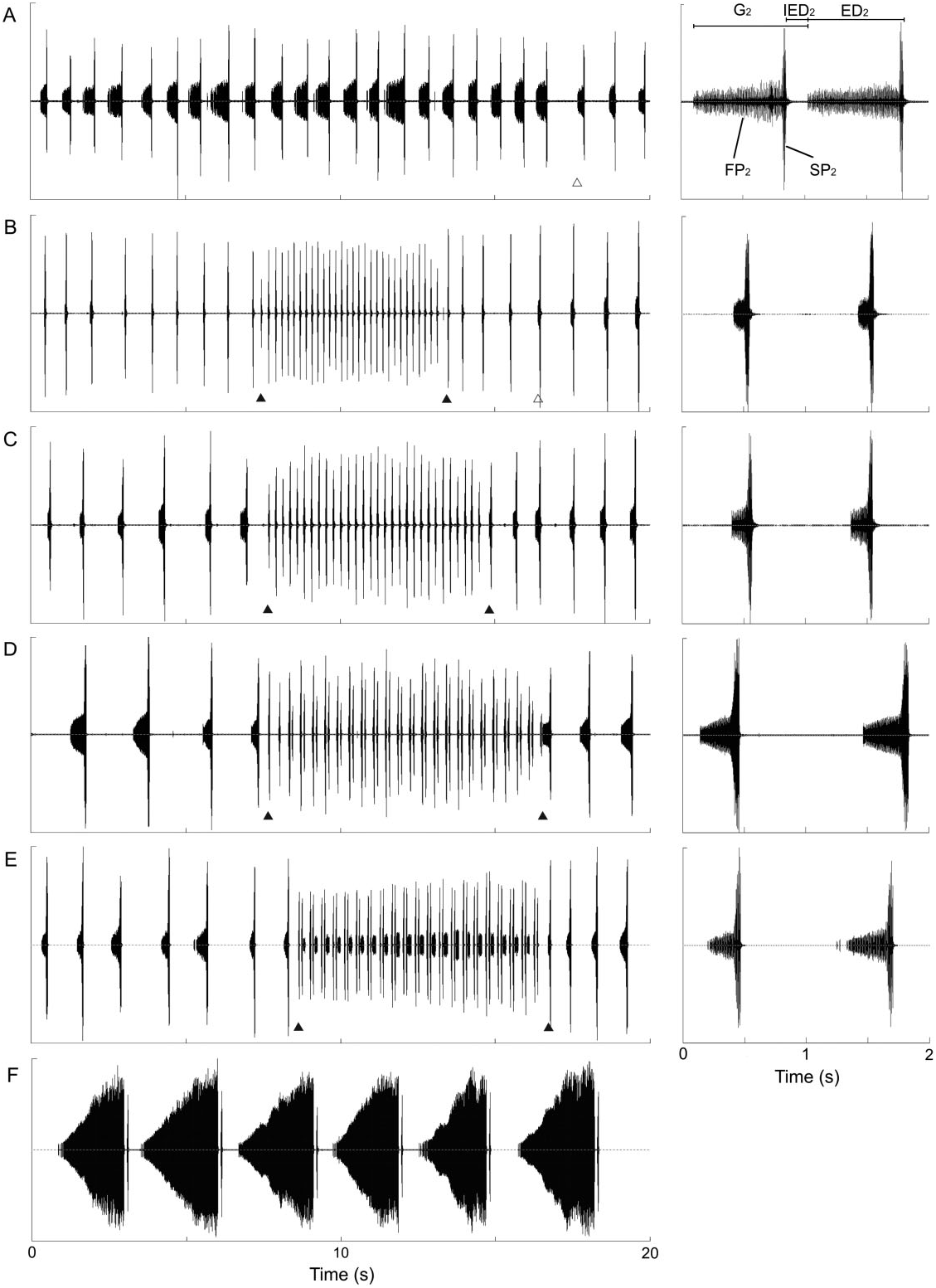
The calling song of C. a. lucana ssp. nov. is intermediate to the songs of C. cerdaniensis s.str. and C. sibillae sp. nov., on the one hand, and C. a. anapaistica on the other hand. It is distinguished by the characteristicly grouped short echemes in the third phrase ( Fig. 6D View Figure 6 ). Cicadetta sibillae sp. nov. and C. cerdaniensis s.str. emit evenly distributed (ungrouped) single echemes with no exceptions. The calling song of C. a. anapaistica also contains grouped echemes, but normally with a longer faint echeme at the end ( Fig. 6E View Figure 6 ).
Composition of calling song
Similar to C. sibillae sp. nov., recordings of C. a. lucana ssp. nov. were analysed in detail for a perch temperature range from 23 to 28 °C (eight individuals, T mean = 24.1 °C), and for some song variables in a broader range (another 43 individuals). They were compared in detail with the data set presented for C. sibillae sp. nov. and C. cerdaniensis s.str., as well as with ten older recordings of C. a. anapaistica (that probably match the same temperature range; Appendix S1) and another 46 recordings in some variables (most of which included perch temperature measurements).
A phrase 1 pattern as in C. cerdaniensis s.str. ( Puissant & Boulard, 2000) was never recorded, and similar to C. a. anapaistica ( Hertach, 2011) , seems not to be prominent. Phrase 2 ( PH 2) consists of a longer series of echemes composed of a low intensity part (FP 2) and a completely connected loud short part (SP 2), which is comparable with the main slow phrases in C. cantilatrix , C. cerdaniensis s.str., C. a. anapaistica , and C. sibillae sp. nov. Phrase 3 ( PH 3) is intermedi- ate to C. sibillae sp. nov. / C. cerdaniensis s.str. and C. a. anapaistica . It consists of fast repetitions of grouped short echemes. The echemes are grouped normally in pairs of two (E 3_1 and E 3_2) in C. a. lucana ssp. nov. (pattern ‘luca_norm’; Figs 6D View Figure 6 , 11 View Figure 11 ). Phrase 2 and phrase 3 are alternating, often for several minutes. The song never starts or ends with phrase 3.
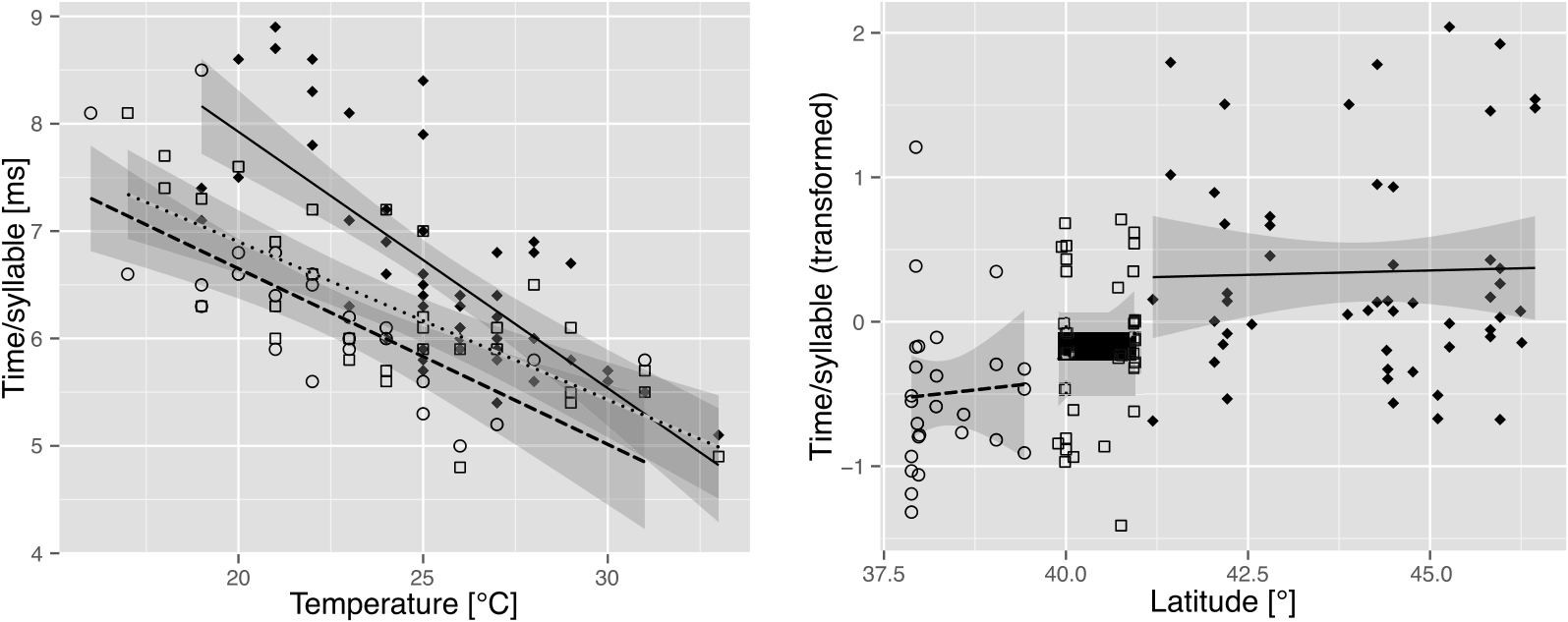
Measurements of the calling song characters in C. a. lucana ssp. nov. within the 23–28 °C temperature range are reported in Table 2 View Table 2 . Phrase 2 echemes are longer on average in this subspecies than in C. sibillae sp. nov. and C. a. anapaistica . ED 2 and IED 2 are positively correlated in some individuals, but not in others. G 3 is slightly longer than in C. a. anapaistica , although the group does not contain a longer final echeme. Differences in the number of syllables forming the short echemes of phrase 3 are marginal between the two subspecies: 6.42 ± 1.10 (E 3_1) and 5.14 ± 0.74 (E 3_2) syllables in C. a. lucana ssp. nov. (mean values from 31 individuals) versus 6.05 ± 1.28 (E 3_1) and 5.35 ± 0.79 (E 3_2) in C. a. anapaistica (mean values of 36 individuals). The dependency on the perch temperature is less obvious in both subspecies of C. anapaistica than in C. sibillae sp. nov. Interestingly, this effect seems mainly to be caused by the ability of C. a. lucana ssp. nov. and especially C. a. anapaistica to move the timbals faster than C. sibillae sp. nov., mainly at low temperatures [ Fig. 12A View Figure 12 , ANCOVA, slightly significant model with interaction for C. a. lucana ssp. nov. and C. sibillae sp. nov., F species (1, 81) = 16.1, P species <0.001, F temp × species (1, 81) = 5.1, P temp × species = 0.026, model without interaction for C. a. anapaistica and C. sibillae sp. nov., F species (1, 76) = 35.3, P species <0.001]. Syllable rates are approximately 15–20% faster at a perch temperature of 20 °C, and form a geographical disruption between the two species ( Fig. 12B View Figure 12 ).
Echeme power (EP) is clearly reduced from phrase 2 (SP 2) to phrase 3 (E 3) in both subspecies [2.3 ± 1.5 dB for C. a. lucana ssp. nov. (mean values of 27 individuals) and 3.6 ± 1.6 dB for C. a. anapaistica (mean values of 40 individuals, see also Hertach, 2011)]. This power reduction is significantly higher even for C. a. lucana ssp. nov. compared with C. sibillae sp. nov. (Wilcoxon–Mann–Whitney rank sum test, W = 735, P = 0.006). The frequency domain (EF) is broad and not a suitable delimitation criteria for the taxa investigated here ( Table 2 View Table 2 ).
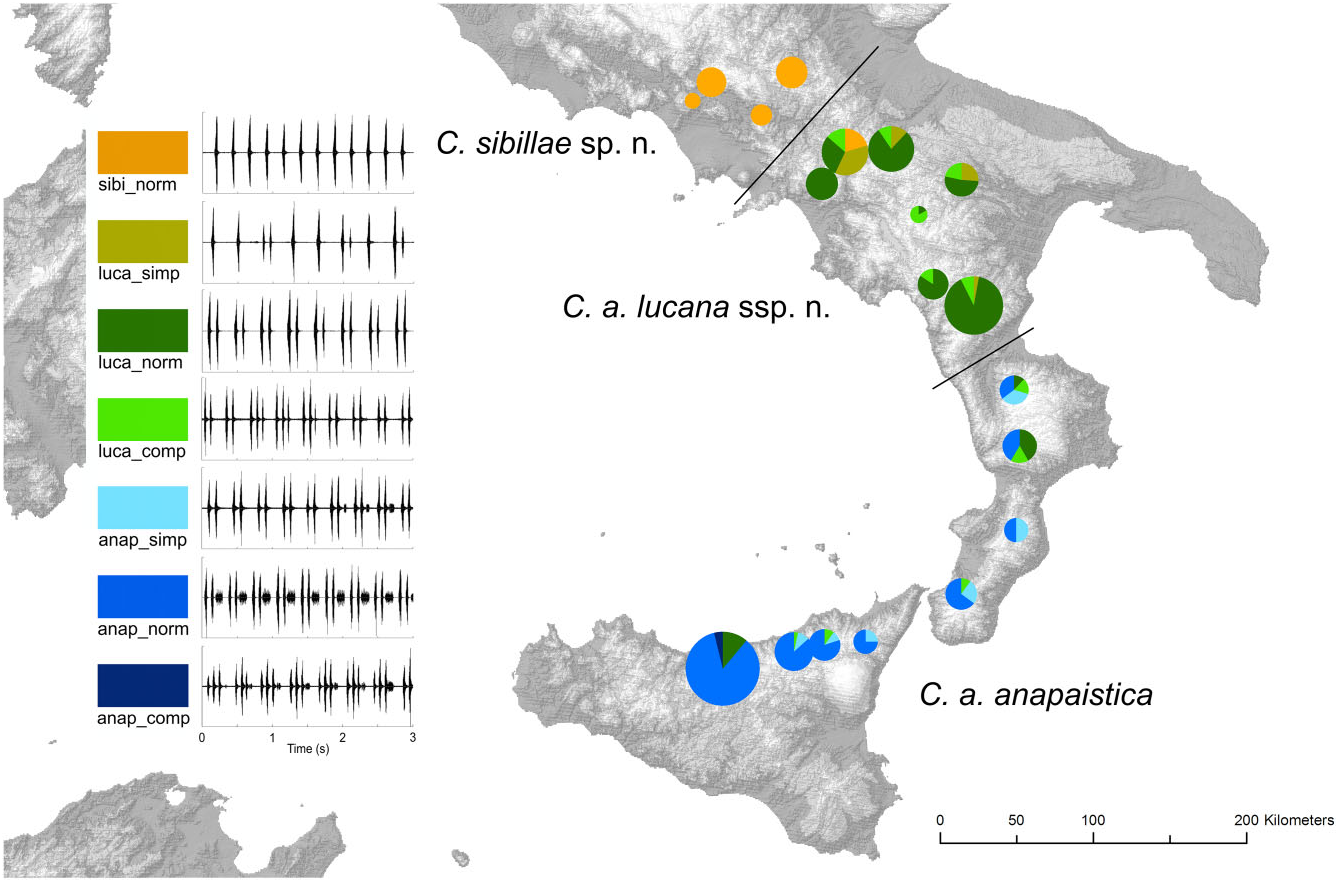
The song of C. a. lucana ssp. nov. is variable in qualitative aspects. Two aberrations must be classified. ‘Luca_comp’ is a more complicated pattern, with three or even four short echemes instead of a pair in phrase 3, produced by a minority of individuals in almost all local populations ( Fig. 11 View Figure 11 ). ‘Luca_simp’ tends to the other direction. In addition to some double echemes a majority of single, ungrouped echemes are emitted. One individual in the Morra de Santis population (Campania) at the northern edge of the distribution area even sang ‘ sibillae ’-typical ungrouped echemes for several sequences and one ‘luca_simp’ sequence. Simplified song structures are also known for C. a. anapaistica ( Hertach, 2011) , and become more and more equal to the C. a. lucana ssp. nov. pattern (‘anap_simp’, ‘luca_comp’, and ‘luca_norm’). They are found in all C. a. anapaistica populations. The percentage of individuals simplifying the structure increases by trend from central Sicily to northern Calabria; however, the transition of local populations capable of emitting the final longer faint echeme is abrupt, and correlated with topography. The gradient is consequently not a typical cline over the whole species range. Populations capable of producing a longer faint echeme in phrase 3 must be assigned to C. a. anapaistica , whereas populations capable of producing solely grouped short echemes are belonging to the new subspecies C. a. lucana ssp. nov.
ETYMOLOGY
The majority of all observations of C. a. lucana ssp. nov. were made in the historical region ‘Lucania’, which is still vernacular in use and is mainly congruent with the current political region ‘Basilicata’. The specific epithet ‘lucana’ is the corresponding adjective in female declination and gives the name to this subspecies.
DISTRIBUTION AND ECOLOGY
Cicadetta anapaistica lucana ssp. nov. is a subspecies endemic to a southern Italian region of approximately 150 km in length and, at most, 100 km wide ( Fig. 9 View Figure 9 ). Local populations reach the southern part of Pollino National Park in the south, the Picentini and Cilento mountains in the west, the Ofanto River drainage basin in the north, and the Difesa Grande Forest near Gravina in Puglia in the east. The distribution is mainly situated in the Basilicata region, but also touches Calabria, Apulia, and Campania. Pollino National Park (San Severino Lucano/Viggianello) contained the most important local population by far ( Fig. 10C View Figure 10 ). Additional significant populations were found at Monte Cupolicchio east of Potenza, at Monte Sirino, at Monticchio Lakes, at Morra de Sanctis, and at Acerno in the Picentini Mountains.
The altitudinal range goes from 250 m asl (Bosco di Monte Orsino, Potenza) to 1960 m asl (Serra di Crispo, Pollino), with a peak of abundance between 800 and 1100 m asl. The maximum altitude observed was exceptionally high for European cicadas. This subspecies was never found near the coast and we only detected very few individuals below 550 m asl. Favourite habitats are mesophilous, extensively used pastures with various bushes, and forest edges or sparse woods [oak ( Quercus spp. ) or beech ( Fagus sylvatica )], with a well-developed understory. In the first habitat type, the new subspecies was regularly observed singing in the herb layer, whereas in the second habitat type it can sing high up in the canopy. Many habitats are fragmented by deforestation, especially in the lower north-eastern parts. Some populations at higher altitudes are threatened by overgrazing, for example in Cilento National Park.
SONG- BASED KEY FOR THE DETERMINATION OF TAXA BELONGING TO THE CICADETTA CERDANIENSIS View in CoL GROUP
(SEE FIGS 6 View Figure 6 , 11 View Figure 11 )
1. Main song element composed by the repetition of echemes with a longer low-intensity part and a completely connected loud short part, all echeme types shorter than 1.5 s ............................. Cicadetta cerdaniensis View in CoL group 2
No echemes existing with a low- and connected high-intensity part or echeme duration longer than 1.5 s .......... ........................................................................... cicada not belonging to the Cicadetta cerdaniensis View in CoL group
2. No fast phrase existing in calling song (but in rarely emitted courtship song).................... Cicadetta cantilatrix View in CoL
Phrase with fast repetitions of echemes existing in calling song ................................................................ 3
3. Fast-repetition phrase composed by evenly distributed (ungrouped) single echemes.......................................4 Fast-repetition phrase characterized by echeme groups............................................................................ 5
4. Central part of fast-repetition phrase composed by echemes with fewer than 6.0 syllables (Pyrenees)................ ................................................................................................................... Cicadetta cerdaniensis View in CoL s.str.
Central part of fast-repetition phrase composed by echemes with more than 6.5 syllables (Apennine and Southern Alps)........................................................................................................... Cicadetta sibillae View in CoL sp. nov.
5. Fast-repetition phrase characterized by echeme groups of solely short echemes (valid at local population level only) ............................................................................................. Cicadetta anapaistica lucana View in CoL ssp. nov.
Fast-repetition phrase characterized by echeme groups finished by a longer faint echeme (valid at local population level only) ............................................................................................ Cicadetta anapaistica anapaistica View in CoL
At lower altitudes, C. a. lucana ssp. nov. occurs syntopically with up to six different species, but not with any other Cicadetta species. At higher altitudes, it is often the single representative of the Cicadidae family. Within the C. montana complex only C. montana s.str. shares the distribution area. This new subspecies was observed in full activity during the first half of July.
Cicadetta anapaistica anapaistica is not restricted to Sicily, as previously stated ( Hertach, 2011). Small and isolated local populations were recently found in the Aspromonte Mountains, in the Calabrian Serre, and in the Sila Mountains ( Fig. 9 View Figure 9 ). They inhabit a narrow ecological niche of more mesophilous habitats than visited before 2013. Additional populations were also found in Sicily, such as in the eastern part of the Nebrodi Mountains and in the Peloritani Mountains. The distribution is significantly larger than expected.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.