Cacotropia (Anastatus) echidna, (Motschulsky)
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4748.3.5 |
|
publication LSID |
urn:lsid:zoobank.org:pub:EE0787C7-147B-4782-BC97-DD80E19F6934 |
|
DOI |
https://doi.org/10.5281/zenodo.3798672 |
|
persistent identifier |
https://treatment.plazi.org/id/C70C87C4-FF8D-9E54-BAB4-14A8FD8CCF1A |
|
treatment provided by |
Carolina |
|
scientific name |
Cacotropia (Anastatus) echidna |
| status |
|
A. (Anastatus) echidna (Motschulsky)
Figs 10–12 View FIGURES 10 View FIGURES 11 View FIGURES 12
Cacotropia echidna Motschulsky, 1863: 57 , fig. 10. Lectotype ♀ [ZMMU, examined] designated by Bouček, 1988: 550.
Cacotropia echidna ; Islam & Hayat, 1986: 65 [catalogue: incertae sedis].
Anastatus echidna ; Bouček, 1988: 550 [generic synonymy].
A. (Anastatus) echidna ; Narendran, 2009: 81, 94 [key].
A. acherontiae View in CoL ; Lalitha et al., 2016, fig. 1 (female) [misidentification].
? Solindenia amara Subba Rao, 1957: 376–378 View in CoL , figs 1–3. Holotype ♀ [INPC, not examined].
? Anastatus amarus View in CoL ; Narayanan et al., 1960: 175 [new combination and key]; Hayat, 1975: 267 [key], 268 [catalogue]; Islam & Hayat, 1986: 58 [catalogue].
? Anastatus amara View in CoL ; Mani, 1989: 681 [key], 687–688 [treatment].
? A. (Anastatus) amarus View in CoL ; Narendran, 2009: 76–77, 95 [key].
? Anastatus acherontiae Narayanan, Subba Rao & Ramachandra Rao, 1960: 171–173 View in CoL , figs 7–9. Holotype ♀ [INPC, not exam- ined].
? Anastatus acherontiae View in CoL ; Hayat, 1975: 266, figs 3A–F, 267 [key], 268 [catalogue]; Mani, 1989: 681 [key], 689 [treatment]; Yang et al., 2015: 161–162 [key and treatment in Chinese], 255 [treatment in English], fig. 82.
? A. (Anastatus) acherontiae View in CoL ; Narendran, 2009: 81, figs 3, 4, 95 [key].
Type material examined. Anastatus echidna : two cards consisting of lectotype and paralectotype female. Lectotype ( Figs 10A, B View FIGURES 10 ) mounted by ventral surface; contorted, missing head and prothorax but with one antenna glued to card in front of specimen, also missing left meso- and metatarsi; original labels: [small square red label] / [small circular yellow label] / “ Cacotropia | echidna | Motchs | Ceyl. [ Ceylon] Mt Patan [Mt. Patannas]” [yellow rectangu- lar card]; subsequent labels: “LECTO- | TYPE” [purple-bordered circular label] / “ LECTOTYPE | ( Cacotropia =) Anastatus | echidna (Motsch.) | det. Z. Bouček, 1980” [rectangular label].
Paralectotype ( Figs 10C, D View FIGURES 10 ) similarly mounted as lectotype; uncontorted; entire except following missing: right antenna beyond fl1, left antenna beyond fl2, left fore leg, and apex of metafemur and base of metatibia (apparently eaten by psocids), with head and mesosoma partly covered in glue and mesoscutum with circular hole on right side; original labels consisting of only red square and yellow circular label, the latter with unrecognizable text, and subsequent labels consisting of two paralectotype labels.
Bouček (1988: 550) stated that of the two female syntypes “the smaller one, with complete antennae and thorax contracted” was selected as the lectotype. Only a single antenna remains, glued to the card such that it must have been intentionally removed from the head. Bouček (1988) did not specifically describe the state of the specimens but because he stated that the lectotype had “complete antennae” this suggests that both antennae were present and the head and prothorax may have been lost some time after he selected the lectotype.
Other material examined. INDIA: Delhi, Delhi— 18.V.1985, J. LaSalle (1♀ CNC); 300m, 10, 18.VII.1990, J. Heraty (2♀ CNC, CNC Photo 2019-58, 2019-59). New Delhi, IARI— 28°37′51″N 77°09′50′E, 220m, 5 (sweep, scrub), 8 (pan trap). XI.2003, J. Heraty (2♀ CNC, one CNC Photo 2019-60); 300m, 11.VII.1990, sweep grass & weeds (2♀ CNC). Delhi, IARI area— 3.X.1979, Bouček, ♀ Anastatus echidna (Motsch.) , det. Z. Bouček 1980, agrees with L-Type ♀ (1♀ BMNH); 3.X.1979, 14.X.1979, Bouček, ♀ Anastatus echidna (Motsch.) , det. Z. Bouček 1980 (2♀ BMNH). H.P . [Himachal Pradesh], Hamirpur, Anu Kalan , 1.VIII.2006, S.M.A. Badruddin & F.R. Khan, ♀ Anastatus acherontiae N. et al., det. Narendran, T.C. 2007 (1♀ CNC) . Kerala, Periyar N.P., 7.X.1979, J.S. Noyes (1♀ BMNH). Punjab, PKT [Pathankot], Dodhpur Kulian , 11.VII.2006, S.M.A. Badruddin, ♀ Anastatus acherontiae N. et al., det. Narendran, T.C. 2007 (1♀ CNC, CNC Photo 2019-64) . Karnataka, Bangalore, 916m, 22–31.V.1986, 1–10.VII.1988, 1–9.IX.1987, K. Ghorpade (3♀ CNC). nr Dharwad , 15°21′05″N 74°53′11″E, 632m, 16.XI.2003, J. Heraty, sw scrub forest (1♀ CNC). E of Hassan, 12°58′36″N 76°14′34″E, 923m, 26.XI.2003, J, Heraty, scrub/sug- arcane (2♀ CNC, CNC Photo 2019-61, 2019-62). Hessaraghatta, Fisheries Div., 27.III.2014, K. Veenakumari, YPT [yellow pan trap] (1♀ CNC). Mandya, 26.IX.2013, K. Veenakumari, MT [Malaise trap] (1♀ CNC) GoogleMaps . Uttar Pradesh, Aligarh , 11.V.1980, 15.III.1981, M. Hayat (2♀ CNC), 28.VIII.1983, S. Islam (1♀ CNC), Anastatus amarus (Subba Rao) ♀, Det. S. Islam. Dehra Dun— 11.V.1928, S.N Chatteriee, ex. Sphingid egg, Dalhbergia, S 1SS00, Anastatus sp. Ch. Ferrière det (1♀ BMNH); 20.X.1979, Bouček, ♀ Anastatus echidna (Motsch.) , det. Z. Bouček 1980 (1♀ BMNH); FRI [Forestry Research Institute], 20.X.1979, S.I. Farouq, Anastatus amarus (Subba Rao) ♀, Det. S. Islam (1♀ CNC). Hathras, Agsauli, 11.III.2013, M.T. Khan, on grasses, Guava orch. [orchard] (1♀ CNC). Etah, Jalesar, 9.III.2013, S.K. Ahmad, on grasses, Mango orch. (1♀ CNC) . PAKISTAN: Punjab, Rawalpindi , VII.1959, ex. Nezara egg (2♀ BMNH) . THAILAND: Doi Inthanon Nat. Park, 70 km SW Chiang Mai, 1260m, 3.I–7.II.1989, T.W. Thormin (1♀ CNC, CNC Photo 2019-63). Loei, Phu Fuea NP, behind check pt, 17°27.829′N 101°121.360′E, 691m, 9–10.XII.2006, Patikom Tumtip, pap trap, T1258 (1♀, QSBG, on indefinite loan to CNC) .
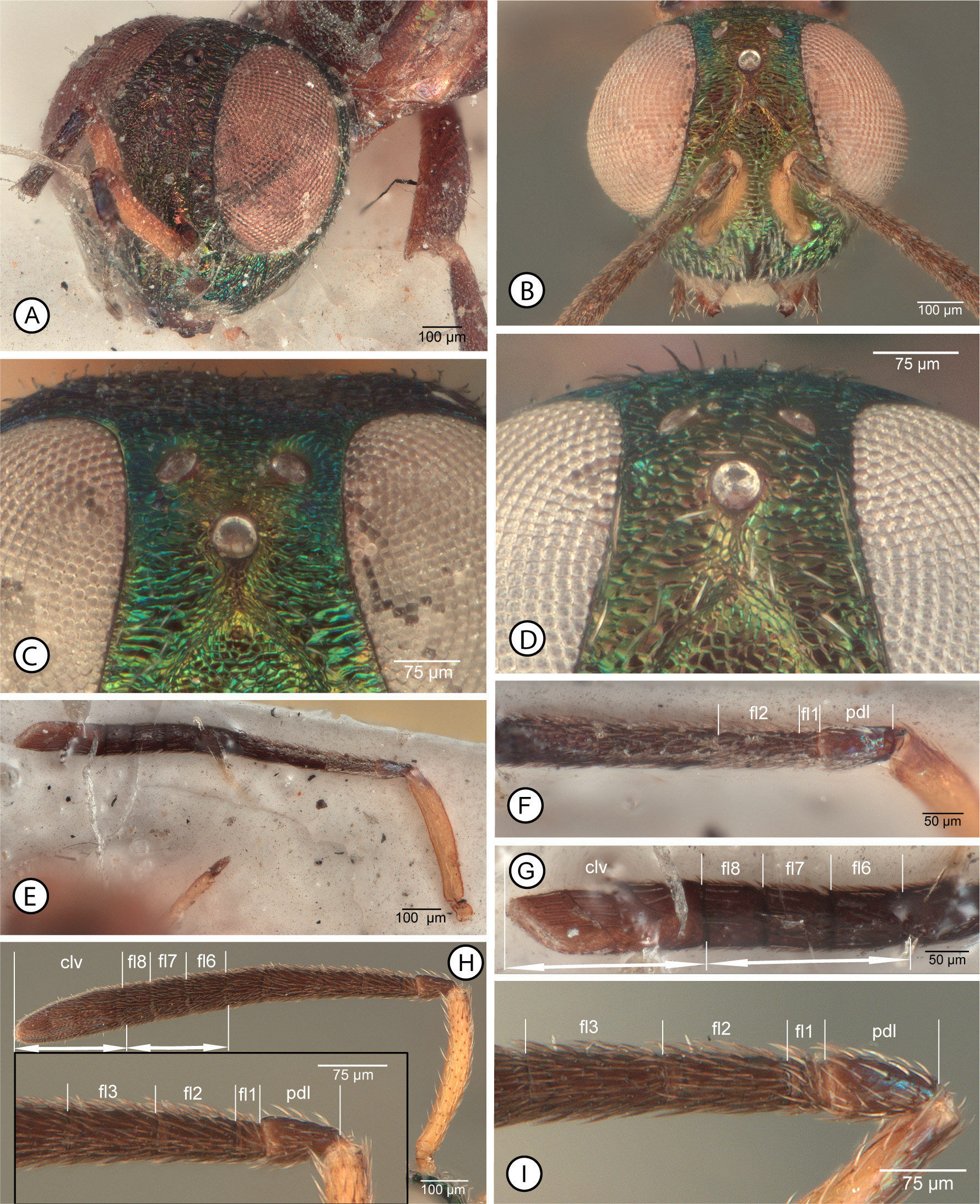
Description of A. echidna type specimens. FEMALE. Length = 6 mm (paralectotype, approximate if head faced vertically). Head ( Fig. 11A View FIGURES 11 ) with frons, upper parascrobal region, and scrobal depression mostly reddish-violaceous with obscure greenish luster under some angles of light, but interantennal prominence, lower face, gena, temples and vertex more distinctly green with limited reddish-violaceous lusters under some angles of light. Antenna ( Fig. 11E View FIGURES 11 ) with scape yellow, pedicel and flagellum brown but pedicel dorsally with bluish to purple luster. Labiomaxillary complex not clearly visible but palpi apparently dark brown. Mesosoma with pronotum mostly covered by head but apparently mostly yellowish to brownish-yellow except for dark arc anterior to each spiracle; mesonotum ( Fig. 12A View FIGURES 12 ) mostly brown in paralectotype with lateral lobes lighter brown, and with slight metallic luster within posterior depressed region of mesoscutal medial lobe, but lectotype ( Fig. 12B View FIGURES 12 ) with more extensive metallic luster, including slight greenish luster on convex part of mesoscutal medial lobe and more distinct bluish to greenish-blue lusters within posterior depressed region of medial lobe and outer surface of lateral lobes posteriorly, and scutellum apparently with slight metallic green or blue lusters; tegula ( Fig. 12A View FIGURES 12 ) and prepectus light brown to brownish-yellow; mesopleurosternum paler than at least medial part of mesonotum, orangish ( Fig. 12D View FIGURES 12 ) to brown ( Fig. 10C View FIGURES 10 ). Metanotum and propodeum mostly concealed ( Fig. 12A View FIGURES 12 ), but apparently dark brown. Fore wing ( Fig. 12E View FIGURES 12 ) with basal cell and costal cell brown basally but otherwise hyaline with white, hair-like setae; discal region extensively brownish-infuscate with slender-lanceolate, brown setae beyond level of base of parastigma except more hyaline and with more hair-like setae apically beyond venation, and with region of paler, more orangish, slender-lanceolate setae ( Figs 10B, D View FIGURES 10 , 12E View FIGURES 12 ) between darker brown setae adjacent to basal fold and darker brown setae basal to anterior and posterior, obliquely oval hyaline region with white, hair-like setae (cf. Figs 12G, H View FIGURES 12 ), the anterior hyaline region extending along about apical half of marginal vein but not quite to base of stigmal vein (cf. Fig, 12G). Front leg ( Fig. 10D View FIGURES 10 ) dark brown except protibia narrowly pale basally and protarsus pale. Middle leg ( Figs 10 View FIGURES 10 B–D) mostly dark brown but with mesofemur paler longitudinally along length over about ventral half, mesotibia narrowly paler basally and apically except for darker apical pegs, mesotibial apical spur pale, and mesotarsus pale except for dark pegs. Hind leg ( Figs 10C, D View FIGURES 10 ) brown except base of metatibia narrowly and metatarsus paler, more yellowish, or basitarsomere brownish basally. Gaster dark brown except Gt1, Gt2 and St1–St3 white and syntergal flange and ovipositor sheaths yellow.
Head ( Fig. 11A View FIGURES 11 ) with exact setal pattern not clearly visible because of condition in paralectotype, but at least scrobal depression bare and interantennal prominence, lower face and gena with white setae; head with HW: HH: HL: IOD = 7.3: 6.8: 4.3: 2.0; OOL: POL: LOL: MPOD = 0.5: 1.2: 1.1: 0.9; eye bare, EH: EW = 4.8: 3.1; scrobal depression carinately margined laterally and dorsally curved toward anterior ocellus, but not margined dorsally, the obscurely delimited dorsal margin separated from anterior ocellus by apparently slightly more than longitudinal diameter of ocellus; frontovertex with vertex and interantennal triangle between posterior ocelli transversely strigose to strigose-imbricate, frons lateral to anterior ocellus and parascrobal region at least dorsally more coarsely transversely strigose, scrobal depression punctate-rugulose to reticulate-rugulose, sculpture of lower parascrobal region, interantennal prominence, and lower parascrobal region obscured by glue but at least lower face lateral of torulus apparently mesh-like reticulate. Antenna ( Fig. 11E View FIGURES 11 ) with scape tubular, slightly curved; dorsal length(lateral width) of scape: pedicel: fl1, fl2 of paralectotype (cf. Fig. 11F View FIGURES 11 ) = 7.6(1.3): 2.0(1.0): 0.6(0.8), 2.1(1.0); length(width) of scape: pedicel: fl4, fl5: fl6: fl7: fl8: clava of lectotype ( Fig. 11G View FIGURES 11 ) = 6.5(1.1): 1.8(1.0): 1.9(1.2): 1.5(1.3): 1.4(1.3): 1.3(1.4): 4.2(1.5). Mandibles normal for genus, with tiny ventroapical tooth and broad truncation.
Mesonotum ( Figs 12A, B View FIGURES 12 ) with convex anterior part of mesoscutal medial lobe with sides subparallel anteriorly and uniformly converging posteriorly to acute angle at about two-thirds length of sclerite, coarsely mesh-like reticulate with white hair-like setae, and posterior depressed portion smooth and shiny with slender, lanceolate, curved white setae, the setal apices mostly directed laterally on either side of midline; mesoscutal lateral lobe finely meshlike coriaceous and bare dorsolongitudinally anterior to posteromedial carinate margin, but with white setae, mostly in one row, along length on outer surface of lateral lobe ( Fig. 12B View FIGURES 12 ); scutellar-axillar complex similarly coarsely mesh-like reticulate as convex part of mesoscutal medial lobe, bare, with scutellum lowly convex ( Fig. 12A View FIGURES 12 ). Acropleuron ( Fig. 12D View FIGURES 12 ) anteriorly, ventrally and posteriorly very finely and inconspicuously mesh-like coriaceous, but dorsally and mesally very finely longitudinally striate to almost smooth. Fore wing ( Fig. 12E View FIGURES 12 ) in uncontorted individual extending to apex of gaster; length about 3.4× width; accurate measurement of vein complex not possible in either female but marginal vein about 4× length of stigmal vein and postmarginal vein about 1.4× length of stigmal vein (lectotype), and costal cell about 1.25× length of marginal vein (paralectotype); costal cell dorsally bare, ventrally with visible setae only within about basal half and apically in front of parastigma; exact setal pattern of basal cell uncertain, but at least hyaline part with row of white setae along mediocubital fold and a few scattered white setae within cell, though apparently mostly bare, infuscate basal part bare, and with at most a few dark setae extending from discal region along extreme posterior margin of wing basally. Profemur with ventral margin evenly curved apically; mesotibia with patch of 5 or 6 mesotibial apical pegs (cf. Fig. 10I View FIGURES 10 ). Metanotum and propodeum concealed under wings but apparently normal for genus, i.e. propodeum with bowie-like plical region.
Gaster normal for genus, about as long as mesosoma; posterior margin of Gt1 incised posteromedially, apparently somewhat more deeply than subsequent tergites; Gt1 and Gt2 smooth and shiny, remaining tergites finely mesh-like coriaceous; tergites beyond Gt2 inconspicuously, sparsely setose; syntergal flange with posterior margin broadly rounded; ovipositor sheaths at most extending only slightly beyond syntergal flange.
Diagnosis for species. Head ( Figs 11 View FIGURES 11 A–D) mostly bright metallic green with variably conspicuous but limited reddish-violaceous luster on upper face and sometimes interantennal prominence; scrobal depression with lateral margins shallowed dorsally such that exact dorsal margin often difficult to determine exactly, but more or less acutely angled dorsomedially and distinctly separated from anterior ocellus by distance equal to about 1.0–2.0× longitudinal diameter of anterior ocellus ( Figs 11C, D View FIGURES 11 ); frons between level of scrobal depression and posterior ocelli mesh-like coriaceous-granular ( Fig. 11D View FIGURES 11 ) to variably distinctly transversely strigose-imbricate ( Fig. 11C View FIGURES 11 ) in contrast to reticulate scrobal depression. Antenna ( Figs 11E, H View FIGURES 11 ) with scape mostly to entirely yellow, at most dark dorsoapically ( Figs 11H, I View FIGURES 11 ), and pedicel and flagellum dark brown; fl2 subequal in length to pedicel ( Figs 11F, H, I View FIGURES 11 ) and apical one to three funiculars quadrate to slightly transverse but combined length of apical three funiculars subequal to clava ( Figs 11G, H View FIGURES 11 ).
Mesosoma. Mesosoma with pronotum ( Fig. 12C View FIGURES 12 ) dark brown to yellowish except for dark arc posterolaterally anterior to each spiracle and, if pale, then pronotal panel ( Fig. 10F View FIGURES 10 : ppl) distinctly lighter in colour than dark prosternum ( Fig. 10F View FIGURES 10 : st1); mesonotum ( Figs 12 View FIGURES 12 A–C) sometimes entirely dark ( Fig. 10G View FIGURES 10 ) but at least scutellum dark with green luster ( Fig. 12C View FIGURES 12 ), though often depressed part of anterior lobe ( Fig. 12C View FIGURES 12 ) and/or mesoscutal lateral lobes ( Fig. 12A View FIGURES 12 ) more brownish and lateral lobes sometimes paler, more yellowish-brown laterally to anterolaterally; scutellum and anteromedial lobe of mesoscutum similarly reticulate; mesoscutal lateral lobe ( Figs 12 View FIGURES 12 A–C) finely coriaceous and bare mediolongitudinally anterior of posteromedial carinate margin relative to sparsely setose outer and inner inclined surfaces of lateral lobe, and depressed posterior part of medial lobe smooth and shiny with slightly lanceolate white setae, the setae projecting laterally, usually over most of surface, but at least to row of more hair-like setae sublaterally so surface much more broadly setose medially than the width of lateral bare region extending to carinate posteromedial margin of lateral lobe ( Figs 12B, C View FIGURES 12 ). Prepectus and acropleuron orangish ( Fig. 10F View FIGURES 10 ) to comparatively dark brown ( Fig. 10C View FIGURES 10 ), but not uniformly dark with metallic luster, the acropleuron often somewhat paler posteriorly than anteriorly below tegula, and anteriorly uniformly setose with hair-like white setae to level about equal with base of fore wing ( Figs 10F, H View FIGURES 10 , 12D View FIGURES 12 ). Fore wing basal and costal cells ( Fig. 12H View FIGURES 12 ) mostly hyaline but infuscate basally, the costal cell bare dorsally but ventrally with complete row of comparatively inconspicuous white setae, and basal cell mostly setose with short white setae except more or less extensively bare behind submarginal vein, at least apically, and with dark setae within basally infuscate part; basal region with dark setae extending from discal region over at least about apical half of vanal area, and cubital area with dark setae or with white setae apically and dark setae basally; discal region ( Figs 12E, F View FIGURES 12 ) mostly brownish-infuscate with slightly lanceolate brown setae becoming more hair-like apically, but with region of paler, more orangish setae behind parastigma and base of marginal vein that extend beyond level of cubital fold to or near posterior margin of wing ( Figs 12 View FIGURES 12 E–H), and with anterior and posterior, oblique-hyaline spots ( Figs 12F, G View FIGURES 12 ) with white hair-like setae behind marginal vein apically, the spots well separated by infuscate region with brown setae and the anterior hyaline spot attaining or almost attaining junction of marginal and stigmal veins. Front leg with profemur and protibia mostly brownish ( Fig. 10H View FIGURES 10 ) or with anterior surfaces variably extensively paler ( Fig. 10F View FIGURES 10 ), but protibia at least narrowly whitish basally, and protarsus with at least apical tarsomeres and usually entirely or mostly pale; profemur without tooth or abrupt angulation ventroapically. Middle leg ( Figs 10F, H View FIGURES 10 ) with at least anterior surface of mesofemur, mesotibia basally and apically, and spur and mesotarsus pale except for dark pegs ( Fig. 10I View FIGURES 10 ), though anterior surface of mesotibia sometimes mostly to entirely pale. Hind leg ( Figs 10F, H View FIGURES 10 ) usually with metafemur and metatibia mostly brown except metatibia with basal or subbasal pale spot, though sometimes metafemur with dorsal surface paler or more extensively pale with only outer surface ventrally brown, and metatibia sometimes with ventral surface paler or more extensively pale with only posterior surface mesally darker brown,
Gaster typical for genus, with basal two sternites ( Figs 10F, H View FIGURES 10 ) and tergites white except Gt1 variably extensively dark basally.
Distribution. * India, * Pakistan, Sri Lanka, * Thailand.
Hosts. Label data of examined individuals indicate eggs of Nezara Amyot & Serville ( Hemiptera : Pentatomidae ) and Sphingidae (Lepidoptera) .
Remarks. Motschulsky (1863, fig. 10) illustrated the contorted lectotype female ( Figs 10A, B View FIGURES 10 ), in which the fore wings appear reduced in size, though in the uncontorted paralectotype ( Figs 10C, D View FIGURES 10 ) they are clearly seen to extend to the apex of the gaster.As a result, Narendran (2009) keyed the species incorrectly, stating that the fore wings extend well beyond T1 but that they are shorter than the gaster. Although the lectotype is contorted and without a head for direct measurements it is the smaller of the two females as is indicated by absolute length measurement of the scape. Because of the condition of the remaining antenna of the lectotype the exact basal and apical limits of the basal flagellomeres is not clearly visible ( Fig. 11F View FIGURES 11 ) so that accurate length measurements are questionable, but fl2 (first funicular sensu Mani 1989, Narendran 2009) appears to be about as long as the pedicel, and fl1 (= anellus) appears to be slightly transverse. In the paralectotype fl2 is definitely slightly longer than the pedicel. The apical three flagellomeres of the lectotype antenna are more clearly visible ( Fig. 11G View FIGURES 11 ), each being subquadrate and together subequal in length with the clava, which is about 3× as long as wide. Condition of the remaining type material also makes exact description of setal patterns of the fore wing costal cell and basal region unreliable. The apparently medially interrupted costal cell setal pattern of the type females as opposed to the complete setal row ( Fig. 12H View FIGURES 12 ) visible in other specimens I identify as A. echidna likely is because not all the setae remain or because of their pale colour they are not visible in the type females.
Using the keys of Narayanan et al. (1960), Mani (1989) or Narendran (2009), A. echidna keys closest to A. acherontiae based on fore wing colour pattern and females having the pedicel slightly shorter than fl2. Narayanan et al. (1960) were the first to recognize that Solindenia amara was incorrectly assigned to genus and correctly transferred the species to Anastatus as A. amarus . They were also the first to differentiate A. amarus from A. acherontiae , primarily by the pedicel purportedly being slightly longer than the first funicular (fl2), which Mani (1989) and Narendran (2009) subsequently repeated. Narendran (2009) also keyed from India five other species with separate anterior and posterior hyaline spots as opposed to a complete hyaline cross band behind the marginal vein— A. alaredactus Narendran (2009 , fig. 8), A. quilonicus Narendran (2009 , fig. 57), A. donius Narendran (2009 , fig. 20), A. mohanae Narendran (2009 , fig. 43), and A. yasumatsui Shafee (1973, fig. 5). Females of the first two species were described as having the fore wings extending at most 0.86 times the length of the metasoma, and thus presumably are not conspecific with A. echidna . Narendran (2009) differentiated A. acherontiae and A. amarus from the last three species by the anterior hyaline spot extending to rather than not extending to the junction of the marginal and stigmal veins, though Shafee (1973, fig. 5) illustrated a fore wing colour pattern for A. yasumatsui that is very similar to that of type material and other specimens I identify as A. echidna ( Fig. 12G View FIGURES 12 ). However, A. yasumatsui and A. echidna cannot be conspecific because females of A. yasumatsui were described as having the mesosoma entirely metallic with bluish-green reflections. Narendran (2009) described both A. donius and A. mohanae as having a more or less similar mesosomal colour pattern as A. echidna , but the fore wings are illustrated with the anterior hyaline spot quite distinctly separated from the base of the stigmal vein in both species ( Narendran 2009, figs 20, 43). This likely also precludes their synonymy with A. echidna , though the anterior hyaline spot often does not quite extend to the base of the stigmal vein in females I identify as A. echidna ( Fig. 12G View FIGURES 12 ). Consequently, of the described species from India whose females are characterized in part by having anterior and posterior fore wing hyaline spots, one or both of A. acherontiae and A. amarus are the most likely potential junior synonyms of A. echidna . Of the three I have seen specimens identified as A. amarus by S. Islam, A. acherontiae by T.C. Narendran, and A. echidna by Z. Bouček, as listed above. Although the females identified as A. acherontiae by Narendran are not ones listed as examined in Narendran (2009), they were identified in 2007 and they likely reflect Narendran’s (2009) concept of this species. However, the females identified as A. amarus by S. Islam have the pedicel slightly shorter than fl2 and thus are similar to females identified as A. acherontiae . Narendran (2009: 77) stated that A. acherontiae and A. amarus may be “sibling species”. In addition, one BMNH female labelled “Lucknow, 21.VIII.1976, LW 390, ex. sugar cane bug, C.I.E. [Commonwealth Institute of Entomology] A9389, NHMUCK 010838808, ♀ Anastatus sp. cf. echidna (Motsch.) + acherontiae Na SR RR, det. Z. Bouček, 1977”, apparently was compared by Bouček with both A. echidna and A. acherontiae and is definitely different from what I interpret as A. echidna . This and three other females from India in the CNC resemble A. echidna but in colour pattern differ most conspicuously in having the prosternum similarly as pale (except narrowly posteriorly along the base of the procoxae) as the pronotal panel; they also differ conspicuously in two sculptural features—the frons is reticulate (i.e. cells are concave because defined by raised ridges) rather than coriaceous (i.e. cells are flat to slightly convex because defined by impressed lines, Fig. 11D View FIGURES 11 ) to transversely strigose-imbricate ( Fig. 11C View FIGURES 11 ), and the mesoscutal lateral lobe is setose anterior to the posteromedial carinate margin rather than bare ( Figs 12B, C View FIGURES 12 ). Another CNC female from India has a similar body colour pattern and more or less mesh-like coriaceous frons as some A. echidna , but differs in not only having the mesoscutal lateral lobe anterior to the posteromedial carinate margin setose but also distinctly roughened, reticulate-imbricate, and the fore wing has the anterior hyaline spot more distinctly separated from the base of the stigmal vein; further, although dark setae separate the anterior and posterior hyaline spots, the intervening region is only slightly infuscate so that the anterior and posterior hyaline spots appear almost continuous, and basally the infuscate region has uniformly dark setae, lacking the region of paler, more orangish setae characteristic of A. echidna ( Figs 12 View FIGURES 12 E–H). A third female from India (CNC) is also similar in body and fore wing colour pattern to A. echidna , including having a differentiated region of more orangish setae basal to the fore wing hyaline spots, plus a meshlike-coriaceous frons and mediolongitudinally bare and finely coriaceous lateral lobes, but has the depressed part of the mesoscutal medial lobe much more narrowly and sparsely setose, the fore wing basal cell almost completely bare, and the anterior hyaline fore wing spot somewhat more widely separated from the base of the stigmal vein than other females I identify as A. echidna . Additional specimens are required to more confidently determine whether this female is A. echidna or belongs to a separate but similar species. I have also seen females (BMNH, CNC) of two other species from India that are more or less similar to A. echidna in fore wing colour pattern and a more or less coriaceous frons, but are readily differentiated by having an entirely dark mesosoma with metallic luster. One is quite similar in fore wing colour pattern to A. echidna except for lacking the region of more orangish setae basal to the hyaline spots; among other features it is differentiated from all other discussed species by having the depressed part of the mesoscutal medial lobe only sparsely setose with white, posteriorly directed setae. Based on its colour pattern, including mostly dark legs, it is possible that this is A. yasumatsui , but Shafee (1973) did not describe setal pattern of the mesoscutum. The second species with an entirely dark mesosoma has the posterior depressed part of the mesoscutal medial lobe setose more similarly to the other described species, but has the mesoscutal lateral lobes uniformly setose anterior of the posteromedial carinate margin. Further, its fore wing colour pattern differs in that only the anterior hyaline spot has white setae, which is distinctly separated from the base of the stigmal vein, and although the hyaline region continues obscurely to the hind margin of the wing all the setae posterior of about the middle of the wing are dark.
I was unable to obtain for study type material of A. amarus or A. acherontiae . Although Narendran (2009) described the head of both A. amarus and A. acherontiae as “reticulate” this is as likely a difference in descriptive format as a true structural difference relative to my observation of the frons being coriaceous to reticulate-strigose in what I identify as A. echidna . All females identified by T.C. Narendran and S. Islam as either A. amarus or A. acherontiae that I examined are A. echidna under my concept, as is the female illustrated in Lalitha et al. (2016, fig. 1). Narendran (2009) separated A. amarus and A. acherontiae in his key to species by several features in addition to relative length of the pedicel. All the stated differential features are colour or structure differences that could form a continuum among females, but the above discussion demonstrates that there are females of other species from India that are similar to E. echidna females in various respects. Consequently, even though it is quite possible, if not probable, that one or both of A. amarus and A. acherontiae are junior synonyms of A. echidna , formal synonymy is not advisable without examination of type material of the former two names. If the names are synonymous then A. echidna is more widely distributed with more hosts than known at present. Although A. amarus is recorded from India only, A. acherontiae was also reported from China by Yang et al. (2015). Anastatus acherontiae is reported as a parasitoid of Pentatomidae and Sphingidae , as for A. echidna , whereas A. amarus is reported as a parasitoid of Pentatomidae and Pyralidae (Lepidoptera) .
| CNC |
Canadian National Collection of Insects, Arachnids, and Nematodes |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Cacotropia (Anastatus) echidna
| Gibson, Gary A. P. 2020 |
A. (Anastatus) echidna
| Narendran, T. C. 2009: 81 |
A. (Anastatus) amarus
| Narendran, T. C. 2009: 76 |
A. (Anastatus) acherontiae
| Narendran, T. C. 2009: 81 |
Anastatus amara
| Mani, M. S. 1989: 681 |
Anastatus echidna
| Boucek, Z. 1988: 550 |
Cacotropia echidna
| Islam, S. S. & Hayat, M. 1986: 65 |
Anastatus acherontiae
| Yang, Z. Q. & Yao, Y. X. & Cao, L. M. 2015: 161 |
| Mani, M. S. 1989: 681 |
| Hayat, M. 1975: 266 |
Anastatus amarus
| Islam, S. S. & Hayat, M. 1986: 58 |
| Hayat, M. 1975: 267 |
| Narayanan, E. S. & Subba Rao, B. R. & Ramachandra Rao, M. 1960: 175 |
Anastatus acherontiae
| Narayanan, E. S. & Subba Rao, B. R. & Ramachandra Rao, M. 1960: 173 |
Solindenia amara
| Subba Rao, B. R. 1957: 378 |
Cacotropia echidna Motschulsky, 1863: 57
| Boucek, Z. 1988: 550 |
| Motschulsky, V. de 1863: 57 |