Araucnephia cearensis, Pessoa, Felipe Arley Costa, Ríos-Velásquez, Claudia María & Py-Daniel, Victor, 2012
|
publication ID |
https://doi.org/ 10.5281/zenodo.208783 |
|
DOI |
https://doi.org/10.5281/zenodo.6170159 |
|
persistent identifier |
https://treatment.plazi.org/id/E64C8794-0034-C227-8FED-FDE70B0BFA12 |
|
treatment provided by |
Plazi |
|
scientific name |
Araucnephia cearensis |
| status |
sp. nov. |
Araucnephia cearensis sp. nov. Pessoa, Rios-Velásquez & Py-Daniel
( Figures 1–69 View FIGURES 1 – 9 View FIGURES 10 – 15 View FIGURES 16 – 22 View FIGURES 23 – 29 View FIGURES 30 – 35 View FIGURES 36 – 41 View FIGURES 42 – 47 View FIGURES 48 – 54 View FIGURES 55 – 60 View FIGURES 61 – 65 View FIGURES 66 – 69 )
Adults: Large, length 2.8 mm (male), 3.5 mm (female). General body coloration blackish brown ( Figs 1 View FIGURES 1 – 9 , 16 View FIGURES 16 – 22 ). Labellum wide ( Figs 10 View FIGURES 10 – 15 , 23 View FIGURES 23 – 29 ). Clypeus prominent and higher than its width, with median longitudinal suture ( Figs 8 View FIGURES 1 – 9 , 17 View FIGURES 16 – 22 ). Wing veins light brown; basal cell present ( Fig. 22 View FIGURES 16 – 22 ); C with spinules and setae; Sc setose in female, bare in male; basal sector of R with hairs arranged in two or three rows, with spine-like setae present on distal one-third. Legs ( Figs 2–4 View FIGURES 1 – 9 ) with light brown coxa with apical dark brown spot and black bristles; light brown femora with basal and apical dark brown spot; basal half of fore- and meso-tibia light brown, with apical halves dark brown; hind tibia light brown; tarsal segments dark brown. Calcipala wider than tall. Pedisulcus absent. Claws with small, triangular-shaped subbasal tooth ( Fig. 24 View FIGURES 23 – 29 ).
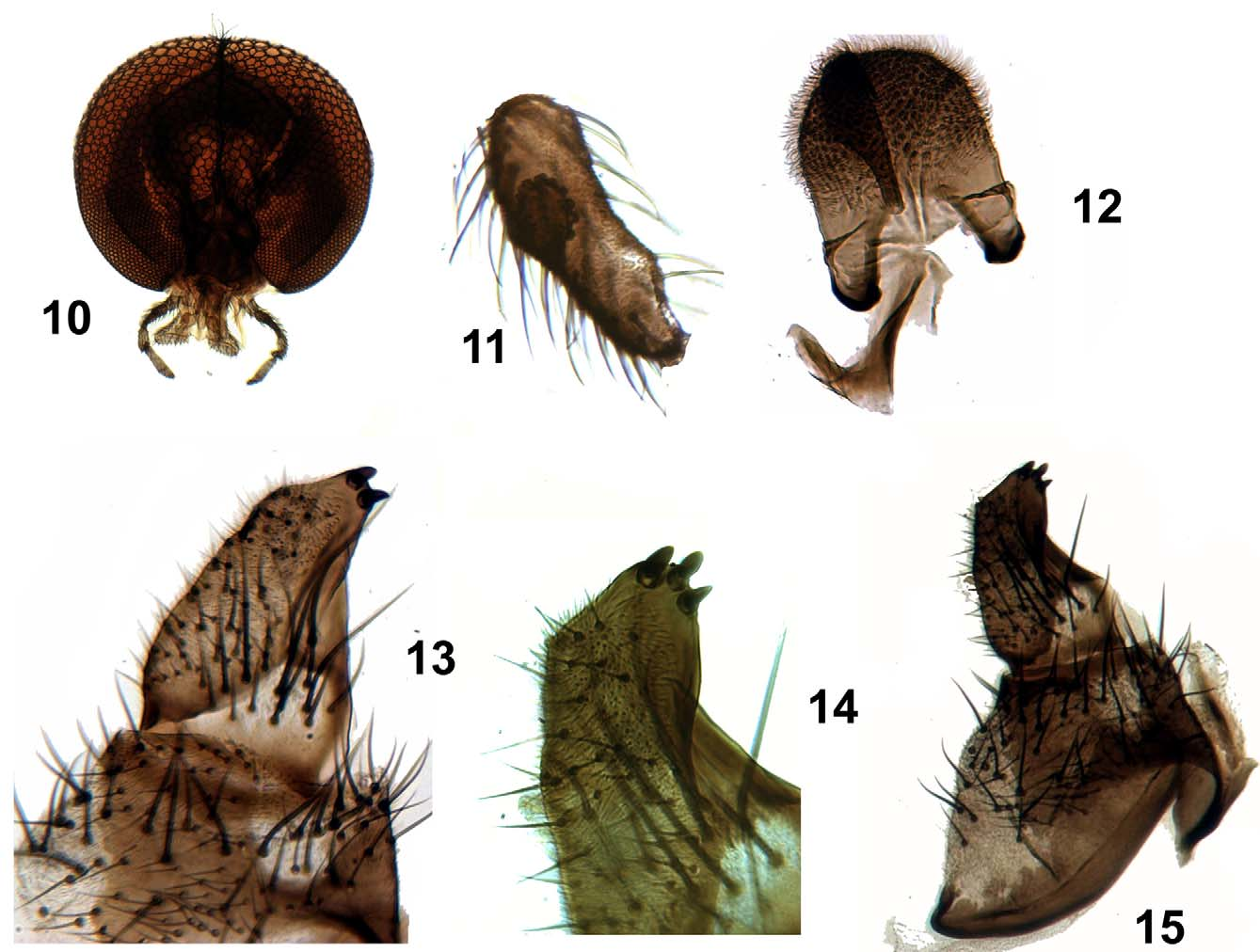
Male: Antenna dark brown, with the apex thinner than the basal area, 0.66–0.68 mm (n = 2) in length; first flagellomere two times higher than its width, whereas other flagellomeres taper apically ( Fig. 5 View FIGURES 1 – 9 ). Ratio of palpomeres 3:4:5 = 1.3:1:1.8 ( Fig. 10 View FIGURES 10 – 15 ). Sensory vesicle of maxillary palpus rounded, covered with tubercles, occupying 2/ 3 of palpomere ( Fig. 11 View FIGURES 10 – 15 ). Wing length = 2.5–2.7 mm; wing width = 1.06–1.32 mm. Scutum dark brown, velvety black posterolaterally, with wide dark brown band dorsomedially. Metanotum velvety black ( Figs 6–7 View FIGURES 1 – 9 ). Katepisternum higher than wide, with complete sulcus. Abdomen dark brown with lateral black stripe on segments VI–VIII. Furcasternum expanded laterally of median branch with abrupt apical constriction ( Fig. 9 View FIGURES 1 – 9 ). Ventral plate rounded. Median sclerite robust, curved, with bilobed plate ( Fig. 12 View FIGURES 10 – 15 ). Gonostylus longer than gonocoxite, subconical, with submedian crest and two or three apical spurs ( Figs 13–15 View FIGURES 10 – 15 ).
Female: Wing length = 2.9–3.06 mm; wing width = 1.16–1.3 mm. Frons, clypeus, antenna, maxillary palpus, and occiput black with grayish pollinosity. Frons width and height equal, with abundant pilosity; frontal angle = 90°; frontocular triangle higher than wide, with long frontal suture ( Fig. 17 View FIGURES 16 – 22 ). Clypeus prominent, higher than wide, with median longitudinal fissure ( Fig. 17 View FIGURES 16 – 22 ). Antenna ( Figure 18 View FIGURES 16 – 22 ) with grayish pubescence, 7.8–8 mm in length; scape and pedicel dark brown, flagellum pale brown, comprised of 9 tapering flagellomeres. Fronto-ocular triangle well developed, without infra-ocular suture ( Fig. 19 View FIGURES 16 – 22 ) Maxillary palpomere (3:4:5) ratios = 1–1.23:1:1.28–1.3 ( Fig. 23 View FIGURES 23 – 29 ); sensory organ about 1/3 of basal article length, with abundant tuberosities ( Fig. 25 View FIGURES 23 – 29 ). Mandible denticulate on both sides with 38-39+1+21–24 teeth; maxilla with 12-13+1+19–20 teeth ( Fig. 25 View FIGURES 23 – 29 ). Basal portion of cibarium smooth, without deep concavity, straight medially, with long sclerotized well-developed cornua and no teeth ( Fig. 26 View FIGURES 23 – 29 ). Scutum and metanotum blackish. Scutum with 1 thin median and 1+1 wider submedian grayish vittae extending longitudinally from anterior to posterior; lateral and posterior margins and humeri blackish brown; prescutellar hairs black, recumbent; pleura blackish brown; scutellum black, with interspersed recumbent setae ( Figs 20–21 View FIGURES 16 – 22 ). Katepisternum higher than wide. Halteres light brown. Legs similar to those of male. Abdomen blackish with grayish pollinosity; tergal plates velvety and darker than remainder of abdomen; pleura glabrous with grayish pollinosity; cerci with rounded margin; paraproct also rounded and slightly higher than wide ( Fig. 27 View FIGURES 23 – 29 ); genital fork with thin, apically expanded median branch equal in length to lateral branches; ( Fig. 28 View FIGURES 23 – 29 ); spermatheca subovoid, with duct, junction hyaline; spinules present ( Fig. 29 View FIGURES 23 – 29 ).
Pupa: cocoon length 6.3 mm (n = 4, mean = 5.9–6.7); total pupa length = 6.1 mm (n = 4, mean = 5.7–6.2 mm); gill length = 1.9–2.86 mm (n = 4, mean = 2.65). Cocoon shapeless, not compact, with evident threads of soft woollike fiber, covering pupa up to gills ( Fig. 30 View FIGURES 30 – 35 ). Head of pupa and body dark brown ( Fig. 31 View FIGURES 30 – 35 ). Frontoclypeus and thorax without tubercles ( Figs 32–34 View FIGURES 30 – 35 ); frontoclypeus with 3+3 small, simple, variably positioned epicraneal trichomes. Thorax with 6–8 single small trichomes per side. Gills short, less than 1/3 of pupal length, with 27–35 terminal branches organized as short basal trunk, with five short and one long primary branch carrying 15 secondary branches ( Fig. 35 View FIGURES 30 – 35 ). Abdomen hard, sclerotized, with numerous small sternal platelets ( Fig. 36 View FIGURES 36 – 41 ); onchotaxy typical of genus, with dorsal simple or bifid setae and ventral simple setae ( Figs 36–38 View FIGURES 36 – 41 ). Sternites with basal spine comb; all sternites, except VII, with small rounded tubercles; sternites V to VII medially divided by longitudinal striate sternal membrane; sternite III with thin setae. Tergites V to IX with rows of large anterior spines; tergites III and IV with 4+4 small posterior hooks ( Figs 37, 39 View FIGURES 36 – 41 ); tergite IX with two long terminal spines and looped trichomes ( Figs 40–41 View FIGURES 36 – 41 ).
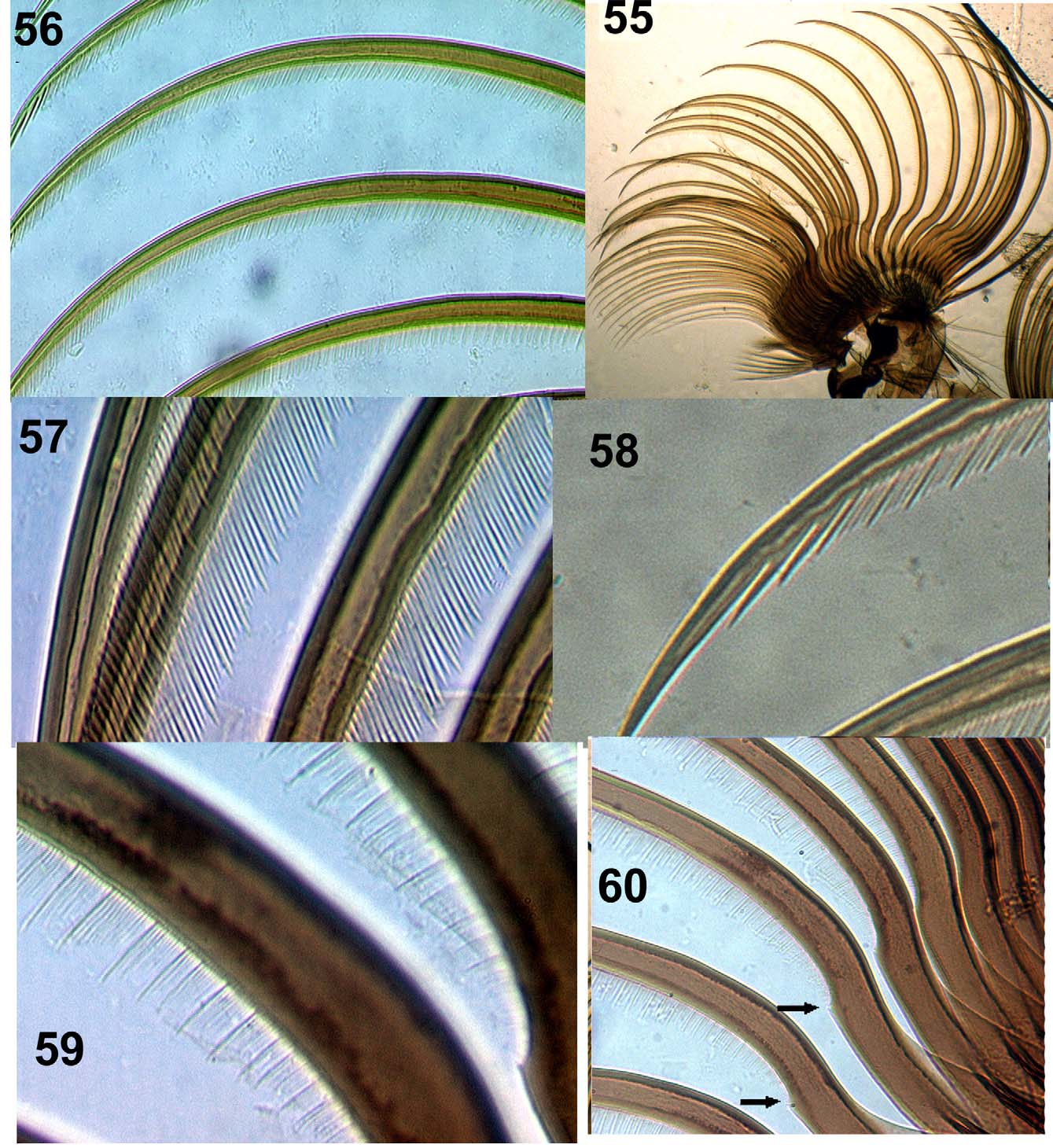
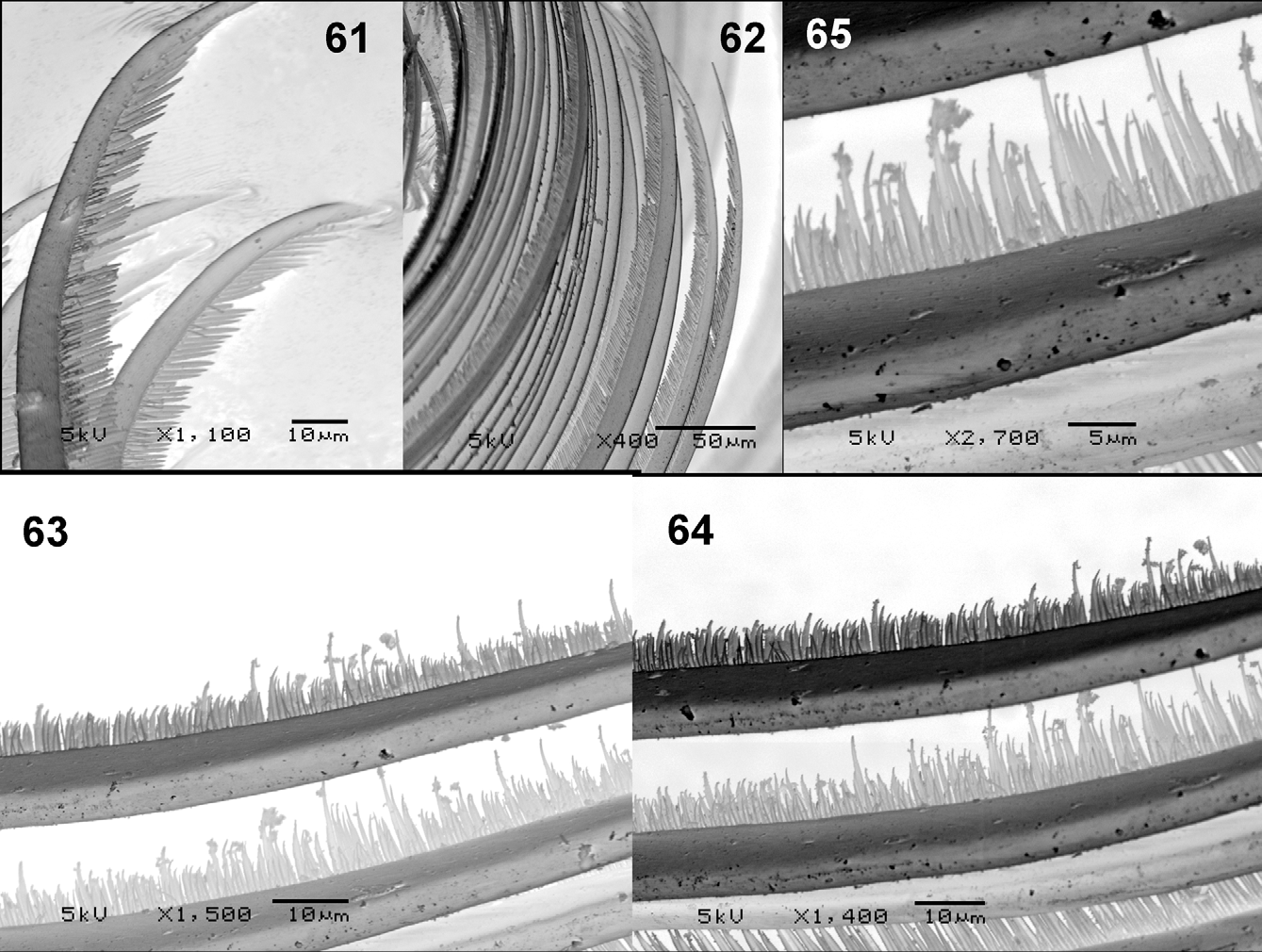
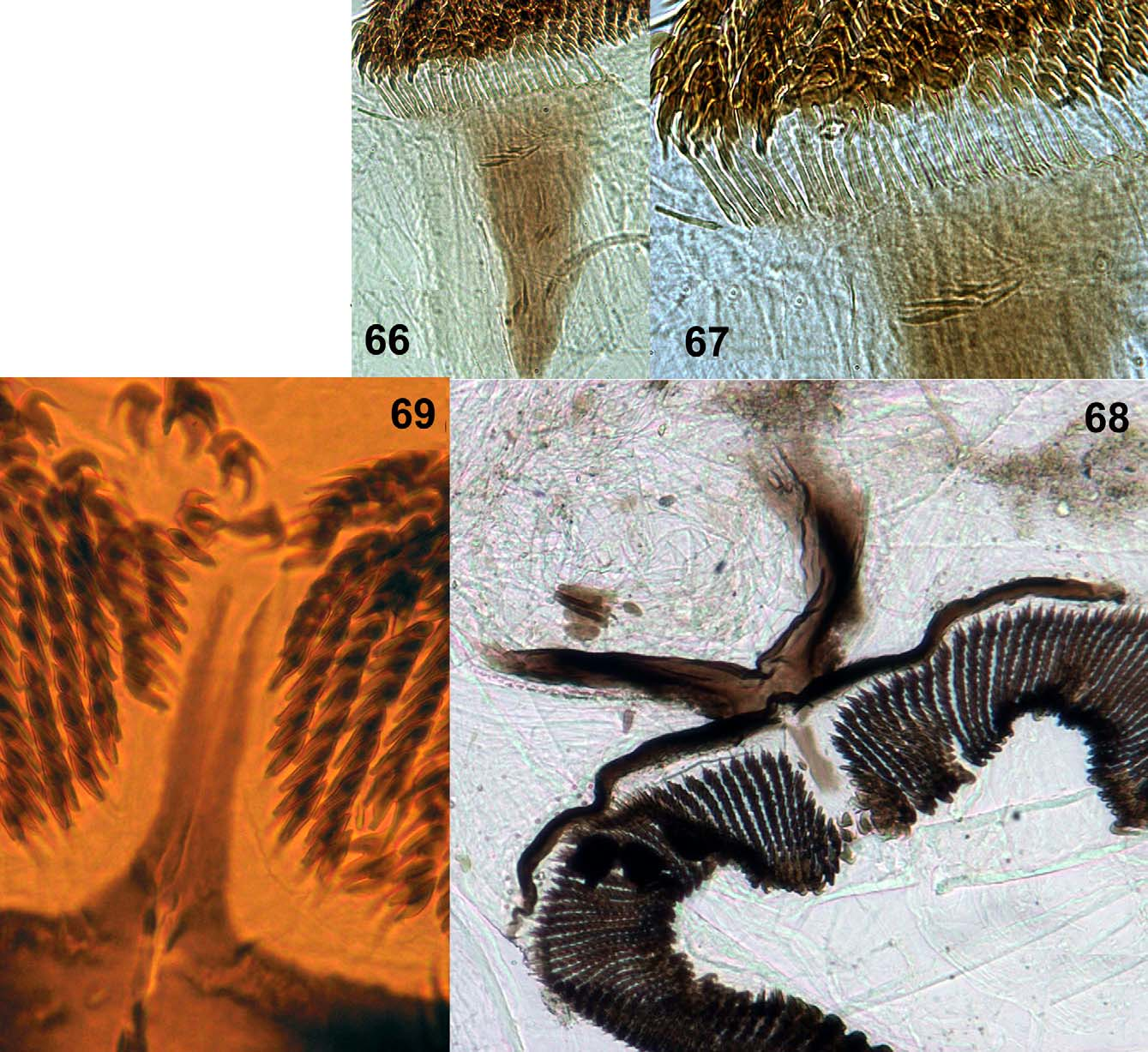
Larva: maximum length = 7.9–9 mm (n = 5). Color, light grayish brown ( Fig. 42 View FIGURES 42 – 47 ). Head light brown with positive spots and very evident ornamentation, with darker area around ocular spot; labral apotome spots dark brown with basal transverse spot, one anteromedial and one posteromedial head spot, and negative anterolateral head spots ( Fig. 43 View FIGURES 42 – 47 ). Cervical sclerite dark brown, wide, elliptical, and free of membrane ( Fig. 44 View FIGURES 42 – 47 ). Postgenal cleft subtriangular and wider basally. Ratio of hypostomium/hypostomial bridge/postgenal cleft = 1:0.7–0.97:0.8–0.97 ( Fig. 45 View FIGURES 42 – 47 ). Hypostomium with one prominent median tooth, three less prominent and one reduced sublateral teeth, one lateral tooth, and three paralateral teeth ( Figs 48–49 View FIGURES 48 – 54 ), and 5–9 lateral serrations per side. Hypostomium with one or two irregular rows of 6–8 long, lateral setae per side, with 3–3 short setae posteriorly near hypostomal groove ( Fig. 49 View FIGURES 48 – 54 ). Antennae shorter than stem of labral fan ( Fig. 43 View FIGURES 42 – 47 ). Antennal articles sclerotized; ratio of antennal articles I–III = 1:1.6–2.3:2.7–3.08 ( Figs 50–51 View FIGURES 48 – 54 ). Basal portion of second segment pale, with other parts dark. Mandible ( Figs 52–54 View FIGURES 48 – 54 ) with two outer teeth, one apical tooth, and three pre-apical teeth; third pre-apical tooth longer than first and first longer than second; internal teeth arranged in several rows; 8–12 marginal teeth, where first is longest; two accessory teeth on external side sometimes anterior to first or at same level as median internal; presence of small lateromandibular process (LMP) ( Fig. 53 View FIGURES 48 – 54 ). Labral fan with 40–42 rays ( Fig. 55 View FIGURES 55 – 60 ); according to Palmer and Craig (2000) microtrichial classification, comb of rays basally weak complex type ( Figs 59, 60 View FIGURES 55 – 60 , 63–65 View FIGURES 61 – 65 ), standard type apically ( Figs 56–58 View FIGURES 55 – 60 , 61–62 View FIGURES 61 – 65 ), while apex of microtrichiae large and strong, larger than others, resembling harpoon ( Figs 58 View FIGURES 55 – 60 , 61–62 View FIGURES 61 – 65 ). Second very small row of microtrichiae present ( Figs 61–65 View FIGURES 61 – 65 ). Labral fan ( Fig. 60 View FIGURES 55 – 60 ), with poorly developed spine basally; labral fan base/apex ratio = 1:3–4.3; scale fan with 6–7 rays. Labral sclerite with small teeth. Prothoracic proleg sclerite enlarged medially, with ungrouped setae; comb with 15–17 teeth ( Figs 66–67 View FIGURES 66 – 69 ). Anal sclerite X-shaped ( Fig. 68 View FIGURES 66 – 69 ), with single trichomes and abundant scales bearing 4–8 branches; presence of uncommon taxonomically median accessory sclerite between posterior arms of the anal sclerite, projecting among the row of hooks ( Fig. 69 View FIGURES 66 – 69 ). Anal ring with 124–129 rows, each with 19–25 hooks. Anal gill with three long unbranched lobes ( Fig. 46 View FIGURES 42 – 47 ), Ventral tubercles small ( Fig. 47 View FIGURES 42 – 47 ).
Etymology. The species name cearensis refers to the state of type locality, Ceará State, Brazil.
Bionomics. Most of the aquatic forms were collected from rocks while a few were found among deciduous leaves in poorly lit riffles. The stream at the type locality was mostly bedrock bottomed and its flow was reduced during the dry season (width = 0.5 m, depth = 5–20 cm). We sampled the area several times during the dry season and we collected very few larvae, although the small stream had flowing water at all sampling points. In March 2011, we collected more individuals during the rainy season so population size of this species appears to be strongly associated with periods of cooler, wetter weather when the flow, velocity, and turbidity of the stream are increased. We did not find this species in other tributaries of the Gavião River near this small stream, which seemingly had the same physical characteristics. Other Araucnephia species are anthropophilic (Coscarón & Coscarón- Arias 2002, Wygodzinsky & Coscarón 1973b), but none of the collectors (FACP and CMRV) felt any black fly bites in the area.
Type locality: Specimens were collected from a small tributary of the Gavião River, near Erundina Hotel, Balneário Cascatinha Road, Mount Maranguape, approximately 750 masl, Maranguape Municipality, Ceará State, 3°53′27"S, 3441′08"W.
Taxonomic comments: Wygodzinsky and Coscarón (1973) created the genus Araucnephia based upon characters of the female genitalia and mandibles (toothed), pupal trichome patterns, and antenna length and hypostomium structure of the larva. Coscarón and Coscarón-Arias (2002) maintained the genus when they described a new species, A. iberaensis , from Argentina, while in 2007 (Coscarón and Coscarón-Arias) they provided keys for the identification of the species. In 2010, Shelley et al. proposed the synonymy of Araucnephia and Araucnephioides Wygodzinsky & Coscarón with Lutzsimulium d'Andretta Jr & Vulcano , based on the presence of looped thoracic trichomes. Such trichomes are present in A. iberaensis but not in other members of the genus. Thus, the taxonomic limits of Araucnephia and Araucnephioides remain controversial. However, morphological-based phylogenies that consider multiple characters do not group Lutzsimulium , Araucnephia , and Araucnephioides in a single monophyletic clade (Gil-Azevedo & Maia Herzog (2007), Gil-Azevedo 2010). Adler and Crosskey (2012) recognized the genus Araucnephia in their latest World species checklist, so we have also decided to recognize the genus as valid.
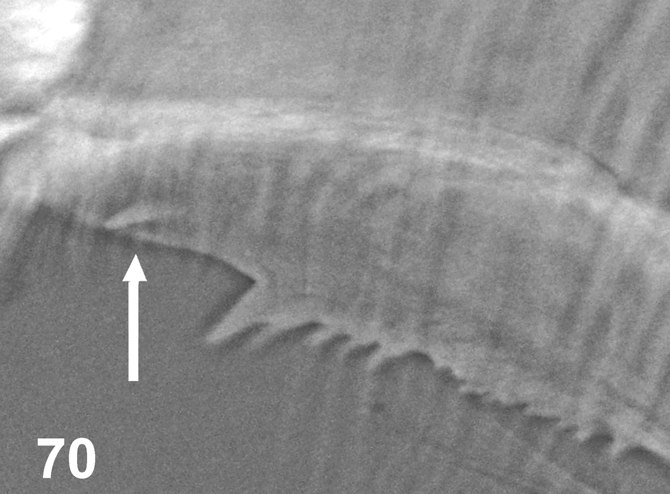
The new species follows the general pattern of Araucnephia as defined by Wygodzinsky and Coscarón (1973), Py-Daniel (1990) and Coscarón and Coscarón-Arias (2002). The pupae can be separated from other Araucnephia pupae based on the number of gills ( A. montana has 14, A. iberaensis has 9, and A. cearensis sp. nov. has 25–35). Another evident autapomorphy of the pupae of A. cearensis sp. nov. is its fully enclosed pupal cocoon, which is shapeless and completely obscures all internal pupal structures. Pupae from the other two described species in the genus Araucnephia have exposed gills. As with A. montana pupae, the frontoclypeus and thorax of A. cearensis sp. nov. lack the platelets present in A. iberaensis pupae. The larva of A. cearensis can be separated from A. montana and A. iberaensis based on its median accessory anal sclerite, which is not present in the other two species in the genus. The lateromandibular process found in A. cearensis sp. nov. is similar to that found in A. iberaensis ( Fig. 70 View FIGURE 70 ), which was not mentioned in the original description although it was drawn partially (Coscarón and Coscarón- Arias, 2002; fig. G, page 86). Slide-mounted mandibles of A. montana larvae lack this structure. Palmer and Craig (2000) correlated the microtrichiae types in black fly labral fans with the type of stream/river they inhabit. Two types of labral fan arrangements were recorded in this new species. These two forms are also present in A. montana , whereas A. iberaensis has only one microtrichial arrangement, i.e., a weak complex form. A possible explanation for the presence of two microtrichiae patterns in both A. montana and A. cearensis sp. nov. species is disruptive selection. Both Araucnephia montana and A. cearensis sp. nov. larvae are found in mountainous regions where the water levels and flow velocities may vary dramatically between the dry and wet seasons. Thus, it is possible that the maintenance of these two very different arrangements within a single species could be a result of different filter-feeding selection pressures, which fluctuate seasonally.
The type locality of A. cearensis sp. nov., i.e., Mount Maranguape, is part of a mountain range that comprises the Brazilian shield. This geological formation is composed of crystalline rocks from the Inferior and Medium Precambrian, which are much older than the Andean mountains (that arose during the Tertiary period). Coscarón and Coscarón-Arias (1995) initially proposed that Araucnephia might be restricted to southern South America because of their high degree of endemism and the strong biogeographic barrier separating the Chilean and the Argentinean Andean regions from the Eastern lowlands. However, they revised this proposal when they described A. iberaensis in the Argentinean lowland ( Coscarón & Coscarón-Arias 2002). The results presented here extend the northern range of the genus Araucnephia beyond an ancient mountain range that arose prior to the Andes. These data agree better with the hypothesis that Araucnephia are derived from a common, widespread Gondwanan ancestor ( Coscarón & Coscarón-Árias, 1995).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.