Hypocreadium picasso, Bray, Rodney A., Cribb, Thomas H. & Justine, Jean-Lou, 2009
|
publication ID |
https://doi.org/ 10.5281/zenodo.187863 |
|
DOI |
https://doi.org/10.5281/zenodo.5633253 |
|
persistent identifier |
https://treatment.plazi.org/id/8B7087CD-FF82-FF81-FF77-FE04CE200CBC |
|
treatment provided by |
Plazi |
|
scientific name |
Hypocreadium picasso |
| status |
sp. nov. |
Hypocreadium picasso View in CoL n. sp.
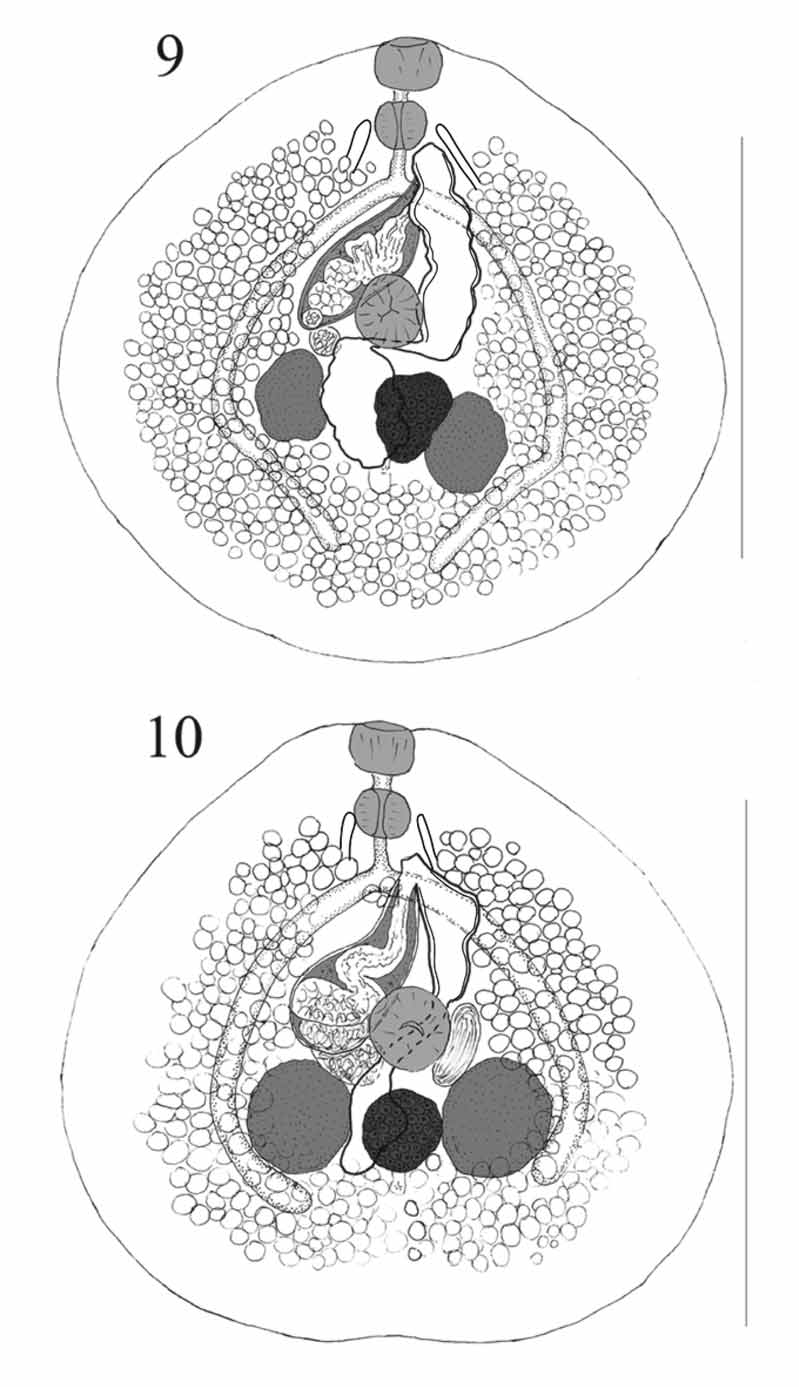
( Figures 9–10 View FIGURES 9 – 10 )
Type-host: Rhinecanthus aculeatus (Linnaeus, 1758) , Balistidae , black-bar triggerfish.
Other-host: Abalistes stellatus (Anonymous) , Balistidae , starry triggerfish.
Site: Intestine.
Type-locality: Lizard Island (14°40’S, 145°28’E, June. 2005).
Other localities: R. aculeatus : Palau (7°21’N, 134°31.47’E, Nov. 2001); A. stellatus: Swain Reefs (21°53’S, 152°21’E, Feb. 2001).
Prevalences: R. aculeatus: Lizard Island 2 of 4 (50%), Palau 1 of 2 (50%); A. stellatus : 1 of 1.
Type-specimens: Holotype QM G 230541, paratypes Lizard Island, QM G 230542 – 230544, BMNH 2009.2.12.17–19; Palau, QM G 230545 – 230546, BMNH 2009.2.12.20–22. Voucher specimen from A. stellatus : QM G 230547.
Etymology: The type-host is also known as the Picasso triggerfish.
Description: Measurements in Table 4 View TABLE 4 . Body about as wide as long, but not quite circular with widest part slightly post-equatorial (broadly pyriform), anterior notch usually absent, very slight notch in one specimen. No tegumental spines seen. Pre-oral lobe absent. Oral sucker wider than long, somewhat angular, usually slightly protuberant from anterior margin, aperture terminal. Ventral sucker rounded, equatorial. Prepharynx usually distinct. Pharynx broadly oval. Oesophagus short, distinct. Intestinal bifurcation in mid-forebody. Caeca narrow, arcuate around gonads, converge posteriorly, end blindly between level of posterior edge of testes and about mid-post-testicular region.
Testes 2, irregularly oval, symmetrical, in anterior hindbody, intertesticular space distinct. External seminal vesicle small, saccular, at level of ventral sucker. Cirrus-sac large, claviform, reaching diagonally across posterior forebody from point dextro-lateral to ventral sucker, reaches over left caecum; surrounded by scattered gland-cells. Internal seminal vesicle oval, not always clearly seen. Pars prostatica bipartite, folded, lined with large anuclear cell-like bodies. Ejaculatory duct muscular, long, highly folded, lined with pavement [or carpet] of cobble-stone like small peduncles. Genital atrium large, often dilated with eggs. Genital pore just sinistral to median line at level of mid-oesophagus (Lizard Island: 9 specimens), posterior oesophagus (Lizard Island: 6; Palau: 1), anterior oesophagus (Lizard Island, 5; Palau, 1) and intestinal bifurcation (Lizard Island, 2; Palau, 1).
Ovary more or less oval to weakly trilobite, intertesticular, always contiguous with sinistral testis and separated (slight or distinct) from dextral testis. Seminal receptacle large, saccular, between sinistral testis and ventral sucker. Laurer’s canal opens dorsally to ovary, not far from excretory pore. Mehlis’ gland anterior to ovary. Uterus passes between ovary and dextral testis to para-ovarian level (Lizard Island, 10 specimens), just into post-ovarian region (Lizard Island, 9; Palau, 1) or is pre-ovarian ( Palau, 2), then proceeds anteriorly, narrowing dorsally to ventral sucker, then widens to form metraterm. Metraterm passes anteriorly, with thick muscular wall and thin sheath of gland-cells, often dilated with eggs. Vitellarium follicular, follicles numerous and relatively large, surround gonads, well separated from body-margins, contiguous and reaching to midway between caecal termination and posterior extremity posteriorly, separated and reaching to about pharyngeal level anteriorly, lateral, ventral and median to caeca, but not dorsal.
Species Hypocreadium patellare Hypocreadium patellare Hypocreadium patellare Form Atypical A Atypical A Atypical B
Host Rhinecanthus verrucosa Rhinecanthus aculeatus Pseudobalistes fuscus Excretory pore at region of posterior part of ovary or just posterior. Vesicle stem very short, divides at level of ovary, arms narrow, reach to level of oesophagus.
Remarks: Molecular results presented by Bray et al. (submitted) indicated the distinctness of the 28S rDNA nuclear and the nad1 mitochondrial gene sequences of Hypocreadium toombo , H. picasso n. sp. and H. patellare from Balistoides viridescens .
This species lacks a distinct anterior notch, in fact, the oral sucker is slightly protuberant and its aperture terminal in most cases. The worm is also rather unusual in shape, in that most Hypocreadium species whose length is similar to its width are basically circular, but these are pyriform. This condition is slight, but clearly recognisable and, apparently, invariant. In the key produced by Bray et al. (1996) this species keys to H. patellare . H. picasso differs in its body-shape, in lacking the anterior notch and in its smaller eggs (46–64 × 23–38 vs 63–81 × 33–43 see Bray & Cribb 1998; Machida & Kuramochi 1999; Yamaguti 1938). The eggs of the specimens from Palau are distinctly smaller than those from Lizard Island.
Hypocreadium patellare ‘Atypical A’, also reported from Rhinecanthus aculeatus (see above), differs from H. picasso n. sp. in being more or less circular with a distinct anterior notch, a distinct pre-oral lobe, a subterminal oral sucker, being slightly larger, lacking a distinct prepharynx, a more anterior genital pore, relatively shorter forebody, larger eggs, and a more posteriorly situated ovary, which is not usually contiguous with the dextral testis (although close).
As far as we are aware, the only species previously reported from Rhinecanthus [as Balistes ] aculeatus is Hypocreadium balistes from the Red Sea ( Nagaty 1942). This species appears to be conventionally circular, but the illustration shows a worm with the lateral and posterior margins folded over, so the shape is not entirely clear, although described as ‘circular’. Going by the original measurements given by Nagaty (1942) H. balistes is distinctly wider than long with the width being 107–118 (111)% vs 94–109% of the length. It also has larger eggs (68–86 × 45–59 (80 × 59) vs 46–64 × 23–38) and apparently grows larger.
Hypocreadium lactophrysi View in CoL from cowfishes and trunkfishes ( Lactophrys View in CoL spp. [ Ostraciidae View in CoL ]) in the Caribbean and Gulf of Mexico is also pyriform ( Nahhas & Cable 1964; Siddiqi & Cable 1960). There is a narrow gap between the vitelline fields and the body margins, the vitelline fields are confluent anteriorly and confluent in the hindbody only as a narrow band of follicles immediately posterior to the ovary, the cuticle is spinous and no prepharynx is found.
TABLE 4. Dimensions of Hypocreadium picasso.
| Species | Hypocreadium picasso | Hypocreadium picasso | Hypocreadium picasso |
|---|---|---|---|
| Host | Rhinecanthus aculeatus | Rhinecanthus aculeatus | Abalistes stellaris |
| Locality | Lizard Island | Palau | Swain Reefs |
| n | 18 | 3 | 1 |
| Length | 550–835 (693) | 549–618 (590) | 690 |
| Width | 544–885 (710) | 564–673 (612) | 596 |
| Forebody | 213–337 (256) | 224–268 (245) | 294 |
| Pre-oral lobe | 0 | 0 | 3 |
| Oral sucker | 46–69 × 64–89 (57 × 76) | 48–61 × 59–74 (55 × 66) | 71 × 75 |
| Prepharynx | 0–21 (11) | 14–17 (16) | 0 |
| Pharynx | 41–61 × 52–72 (52 × 63) | 33–49 × 47–57 (42 × 53) | 42 × 56 |
| Oesophagus | 21–61 (31) | 23–35 (28) | 64 |
| Intestinal bifurcation to ventral sucker | 80–148 (108) | 89–122 (100) | 100 |
| Pre-vitelline distance | 62–232 (92) | 77–95 (89) | 127 |
| Ventral sucker (VS) | 81–136 × 77–126 (100 × 96) | 75–98 × 78–93 (86 × 84) | 103 × 105 |
| External seminal vesicle | 19–74 × 14–56 (33 × 26) | 43–89 × 29–44 (64 × 36) | 97 × 55 |
| Cirrus-sac | 171–275 × 63–126 (211 × 79) | 181–196 × 79–93 (191 × 87) | 198 × 100 |
| Genital pore to ventral sucker distance | 86–196 (126) | 100–124 (113) | 141 |
| VS to Ovary | 9–61 (29) | 19–40 (27) | 19 |
| Ovary | 78–122 × 59–128 (104 × 81) | 60–85 × 64–82 (74 × 71) | 84 × 71 |
| Ovary to closest testis | 0 | 0 | 5 |
| Metraterm | 131–280 (198) | 159–162 (161) | 183 |
| Testes | 94–141 × 73–119 (115 × 92) | 91–119 × 93–113 (110 × 104) | 112–118 × 93–96 |
| Distance between testes | 82–202 (125) | 68–79 (75) | 88 |
| Post-testicular distance | 134–290 (216) | 149–162 (157) | 200 |
| Post-ovarian distance | 150–269 (215) | 164–165 (165) | 200 |
| Post-uterine distance | 155–260 (209) | 161–231 (204) | 154 |
| Post-caecal distance | 80–202 (139) | 106–132 (120) | 151–165 |
| Post-vitelline distance | 36–79 (52) | 58–75 (69) | 50 |
| Eggs | 55–64 × 25–38 (59 × 32) | 46–51 × 23–31 (48 × 27) | 68 × 34 |
| Width as % of body-length | 94.0–109 (102) | 99.2–109 (104) | 86.3 |
| Forebody as % of body-length | 31.4–40.8 (36.9) | 40.4–43.3 (41.6) | 42.5 |
| Sucker-length ratio | 1:1.56–2.20 (1.76) | 1:1.52–1.60 (1.56) | 1:1.45 |
| Sucker-width ratio | 1:1.14–1.56 (1.27) | 1:1.12–1.59 (1.30) | 1:1.39 |
| Pharynx: oral sucker ratio | 1:1.12–1.30 (1.20) | 1:1.21–1.29 (1.24) | 1:1.35 |
| Cirrus-sac length as % of body-length | 26.7–35.9 (30.7) | 30.0–35.8 (32.5) | 28.6 |
| VS-Ovary as % of body-length | 1.27–8.92 (4.18) | 3.14–6.70 (4.57) | 2.71 |
| Post-testicular distance as % of body-length | 24.0–35.8 (31.2) | 25.7–27.1 (26.6) | 29 |
| Post-ovarian distance as % of body-length | 26.7–35.0 (30.9) | 26.7–30.0 (28.0) | 29 |
| Pre-vitelline distance as % of body-length | 9.31–28.1 (13.2) | 14.1–15.7 (15.1) | 18.5 |
| Post-vitelline distance as % of body-length | 6.34–10.3 (7.53) | 10.7–12.5 (11.7) | 7.24 |
| Post-uterine distance as % of body-length | 21.8–35.1 (30.3) | 26.0–40.3 (34.9) | 22.4 |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
SubClass |
Digenea |
|
Order |
|
|
Family |
|
|
Genus |