Coprophanaeus (Metallophanaeus) machadoi ( Pereira & d’Andretta, 1955 ), Pereira & d'Andretta, 1955
|
publication ID |
https://doi.org/10.11646/zootaxa.3869.4.7 |
|
publication LSID |
lsid:zoobank.org:pub:7446EA9F-1C54-47AB-ABF1-0A82B1E59687 |
|
DOI |
https://doi.org/10.5281/zenodo.5457226 |
|
persistent identifier |
https://treatment.plazi.org/id/FA458A0F-FFFC-FFC4-FF44-57533F84FCBD |
|
treatment provided by |
Plazi |
|
scientific name |
Coprophanaeus (Metallophanaeus) machadoi ( Pereira & d’Andretta, 1955 ) |
| status |
|
Coprophanaeus (Metallophanaeus) machadoi ( Pereira & d’Andretta, 1955) View in CoL , revalidated
Figs. 1–9 View FIGURES 1 – 4 View FIGURE 5 View FIGURES 6 – 11 , 12–13 View FIGURE 12 – 13 .
Phanaeus (Metallophanaeus) machadoi Pereira & d’Andretta, 1955: 257 View in CoL –260, figs. 14–17; Edmonds & Zidek, 2010: 29.
Coprophanaeus (Metallophanaeus) machadoi View in CoL : Vaz-de-Mello, 2000: 192; Arnaud, 2002: 56; Biodiversitas, 2007: 123; Edmonds & Zidek, 2010: 2, 31.
Phanaeus saphirinus: sensu Harold, 1875: 42 View in CoL .
Phanaeus (Metallophanaeus) saphirinus: sensu Edmonds, 1967: 97 View in CoL –104.
Coprophanaeus (Metallophanaeus) saphirinus: sensu Edmonds & Zidek, 2010: 30 View in CoL View Cited Treatment , fig. 71.
Type specimen: Holotype: male ("Holotipo", " Phanaeus machadoi sp. n. ♂, P. Pereira det. 955", "Açucena, Mg, 5.II. 952, A. Machado", "26153"), MZSP (examined) ( Figs. 5–6 View FIGURE 5 View FIGURES 6 – 11 ).
Type locality: Açucena, Minas Gerais, Brazil ( Pereira & d’Andretta, 1955).
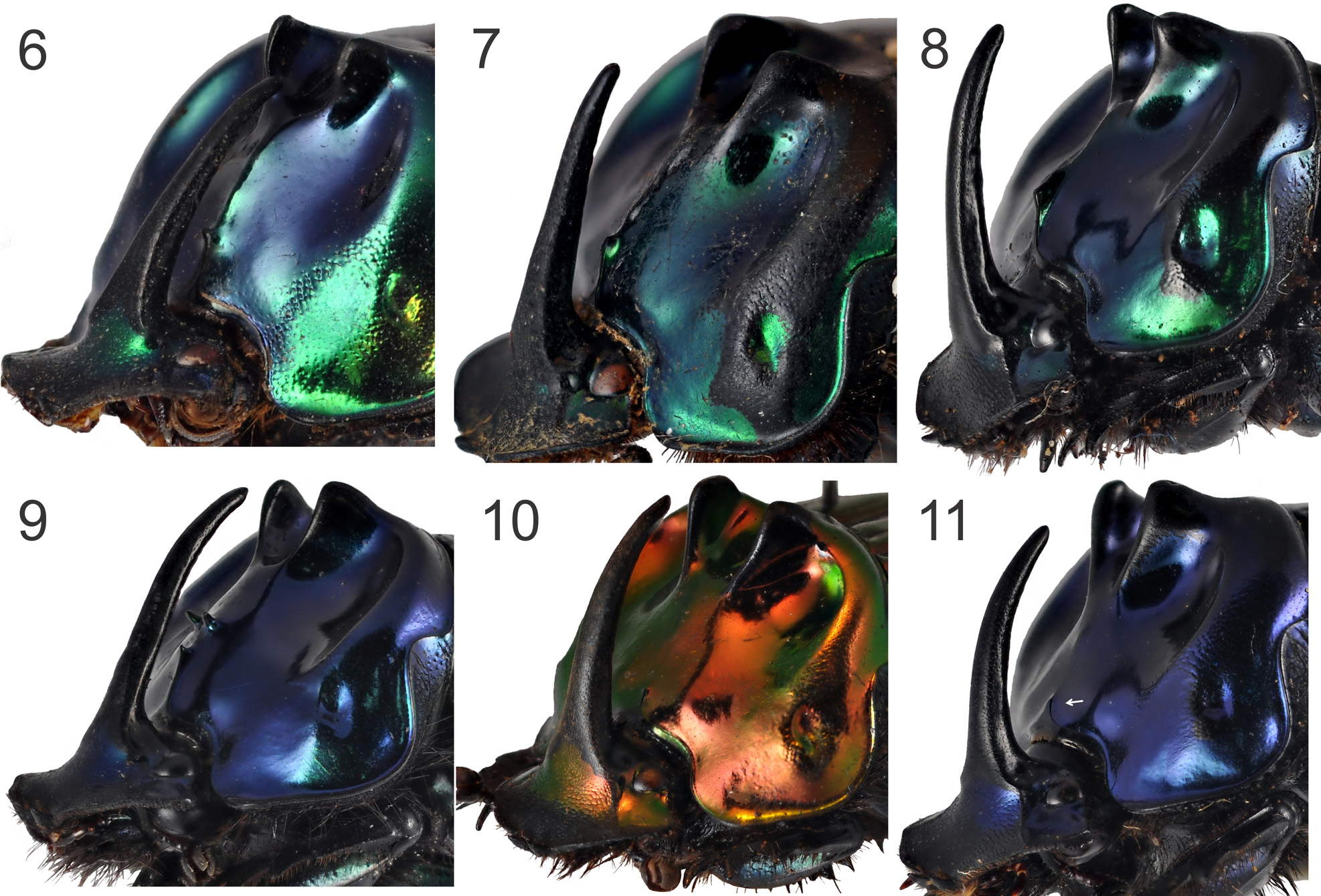
Description: General —Paraocular areas (genae) not carinate lateral to eyes. Pronotum smooth posteromedially, or, in minor specimens, sparsely punctured; posterior angle slightly and triangularly produced posteriorly; posterior fossae absent and sulcus paralleling posterior margin of pronotum effaced medially. Anteromedian angle of metasternum rounded, not salient; angle capped with elongate thickening usually visible from below as a broad “V”. Elytral interstriae flat; striae simple, very fine, superficial, not impressed, but, in lightcolored specimens, much more clearly visible by their darker coloration (usually dark blue); bases of striae 1-4 distinctly fossate, fossae progressively larger laterally. Ventral surface of protibia, lateral to longitudinal carina paralleling inner margin, entirely punctatorugose. Pygidium lacking basal groove. Color usually shining both metallic green and blue ( Figs. 1–4 View FIGURES 1 – 4 ), but sometimes entirely light green or dark blue; never with metallic red.
Male —Paraocular areas mostly smooth, rugosity present at most only adjacent to outer margin (in minor specimens, rugosity sometimes more widespread, but never as females). Head with evenly tapering long horn, base not abruptly swollen. Pronotum with pair of closely set, apically slightly convergent processes near posterior margin, separated by strong concavity (in minor specimens, processes and concavity progressively reduced); disk anterior to processes with deep, round concavity on each side, or, in minor specimens, only slightly concave or completely absent. In major males, anterior margin of pronotum with long midlongitudinal filiform prolongation, sometimes raised into a sharp and acute ridge; prolongation followed by midlongitudinal well-marked carina ( Figs. 6–9 View FIGURES 6 – 11 ); anterior slope of pronotum sometimes ornamented by pair of divergent tubercles ( Fig. 9 View FIGURES 6 – 11 ). In minor specimens, filiform prolongation progressively shorter or absent, sometimes slightly triangular. Pronotal sculpturing limited to weak rugosity on lateral margins. Parameres only slightly concave laterally; apical processes rounded and with posterior flap only slightly curved ( Fig. 12 View FIGURE 12 – 13 ).
Female —Paraocular areas broadly rugose, smooth only near to eyes. Pronotum with small, weakly trituberculate crest adjacent to anterior margin followed by weak concavity bounded posteriorly by pair of weak tumosities near middle of disk; in minor specimens, crest and concavity reduced and tumosities absent. Pronotum finely granulorugose on disk and sides, punctate posteromedially; in minor specimens, granulorugosity progressively more widespread posteriorly.
Measurements: Males ( 15 specimens): TL: AV: 17.31; MX: 21; MN: 15; SD: 1.89. PW: AV: 10.93; MX: 13; MN: 9.3; SD: 1.25. Females ( 15 specimens): TL: 18.08; MX: 23; MN: 15; SD: 2.42. PW: AV: 10.97; MX: 14; MN: 9; SD: 1.74. Total ( 30 specimens): TL: AV: 17,7; SD: 2.2. PW: AV: 10.95; SD: 1.49.
Intraspecific variation and taxonomic discussion: Coprophanaeus machadoi is a cryptic species widely confused with C. saphirinus and identified in collections and publications with this latter name. In most large collections we have examined ( BMNH, CPFA, MNHN, MNRJ, and MZSP), including that where the holotype is deposited, no specimen (but the holotype) of C. machadoi was identified as such, and individuals of this species were mixed among large series of C. saphirinus . In addition to the type specimen, only a single male was correctly identified in a publication as C. machadoi ( Arnaud, 2002) , but even in that work, other C. machadoi were certainly misidentified as C. saphirinus since Arnaud cited Espírito Santo ( Brazil) as part of the distribution range of the latter species. In fact, part of this confusion is due to the fact that the only two specimens so far correctly identified as C. machadoi lacked the aedeagus and, therefore, the more easily observed characteristics that differentiate the two species had not been detected until now ( Pereira & d’Andretta, 1955; Arnaud, 2002; see below). Furthermore, the lack of distinction between the females of these two species contributes to the cryptic nature of C. machadoi . Nevertheless, two evident morphological characteristics observed by us support the validity of C. machadoi and, being in agreement with the distribution of the two species, make identification easier.
Pereira & d’Andretta (1955) used the presence of a midlongitudinal carina on the anterior slope of male pronotum of C. machadoi to distinguish it from C. saphirinus ( Fig. 6–9 View FIGURES 6 – 11 ). Males C. saphirinus , in general, have no trace of this carina; only in a few specimens there is a very faint longitudinal line marked on the anterior slope of pronotum. In many small males of the C. machadoi , however, the carina may be completely absent and, without locality data, it is more difficult to distinguish them from C. saphirinus . In C. machadoi , anterior to and continuous with this carina, there is a midlongitudinal and filiform prolongation of the anterior pronotal margin that is elevated as a sharp and acute ridge in major specimens ( Fig. 6–9 View FIGURES 6 – 11 ). Minor specimens have this prolongation progressively shorter and triangular, being absent in smaller individuals. In C. saphirinus , regardless of the size of the male, the prolongation may be short and triangular or absent ( Fig. 10–11 View FIGURES 6 – 11 ). In this latter case, the anterior margin is simply rounded.
In one specimen of C. machadoi observed by us, the midlongitudinal carina is accompanied by a pair of divergent tubercles ( Fig. 9 View FIGURES 6 – 11 ). However, the presence of this feature does not appear to be unique to this species, since we observed two large males of C. saphirinus , one from Virgínia (Minas Gerais) and other from Santa Catarina, that have a pair of small elevations in the anterior slope of pronotum that we believe to be a very worn pair of tubercles. Edmonds & Zidek (2010) also observed a pair of tubercles in a specimen identified as C. saphirinus , but since they did not provide the origin of this individual, it is impossible to know if it really was a C. saphirinus or a C. machadoi .
The second distinguishing and most easily observable characteristic of C. machadoi is the shape of the parameres: in C. machadoi , the parameres are only slightly concave laterally and the posterior flap of the apical process is only slightly elevated ( Fig. 12 View FIGURE 12 – 13 ), while in C. saphirinus the lateral curvature is much more profound and the posterior flap of the apical process is strongly elevated and curved ( Fig. 13 View FIGURE 12 – 13 ). Indeed, this is the feature that more reliably differentiates males of both species, regardless of their size.
Pereira & d’Andretta (1955) stated that C. machadoi differed from C. saphirinus mainly by differences of elytral sculpture, with the striae being more strongly marked and the interstriae more distinctly punctured in C. machadoi than in C. saphirinus . Edmonds & Zidek (2010) considered this characteristic and the midlongitudinal carina as individual variations of the C. machadoi holotype within the intraspecific variation of C. saphirinus and thus established the synonymy between these two names. Despite that the elytral striae of the holotype of C. machadoi are really unusually marked (in fact, they are carinulate in a similar manner to those of C. punctatus ), we agree that this is a peculiar individual variation and that the most common condition in C. machadoi is similar to that of C. saphirinus , i.e. with very fine and superficial striae and smooth interstriae. The midlongitudinal pronotal carina and the filiform prolongation of the anterior pronotal margin, on the other hand, are consistently correlated with the structure of the parameres and together these three characteristics support the distinction between C. machadoi and C. saphirinus .
Trends in size and degree of development also support the validity of C. machadoi . Although the total length (TL) and pronotal width (PW) averages are slightly larger in C. saphirinus than in C. machadoi (TL= 17.7 and PW= 10.95, in C. machadoi ; and TL= 19.0 2 and PW= 12.0 9, in C. saphirinus ), the most notable difference is that males of C. machadoi are never as large nor have cephalic and pronotal ornamentation as developed as the largest males of C. saphirinus (TL MX = 21 and PW MX = 13, for C. machadoi males; and TL MX = 23 and PW MX = 19, for C. saphirinus males). The cephalic horn, for example, is strongly curved backwards in some major males of C. saphirinus ( Figs. 11 View FIGURES 6 – 11 , 15 View FIGURE 15 ), while it is generally straight or at most only slightly curved apically in larger C. machadoi ( Figs. 6–9 View FIGURES 6 – 11 ). Size differences are repeated in females (TL MX = 23 and PW MX = 14, in C. machadoi ; TL MX = 24 and PW MX = 19, in C. saphirinus ).
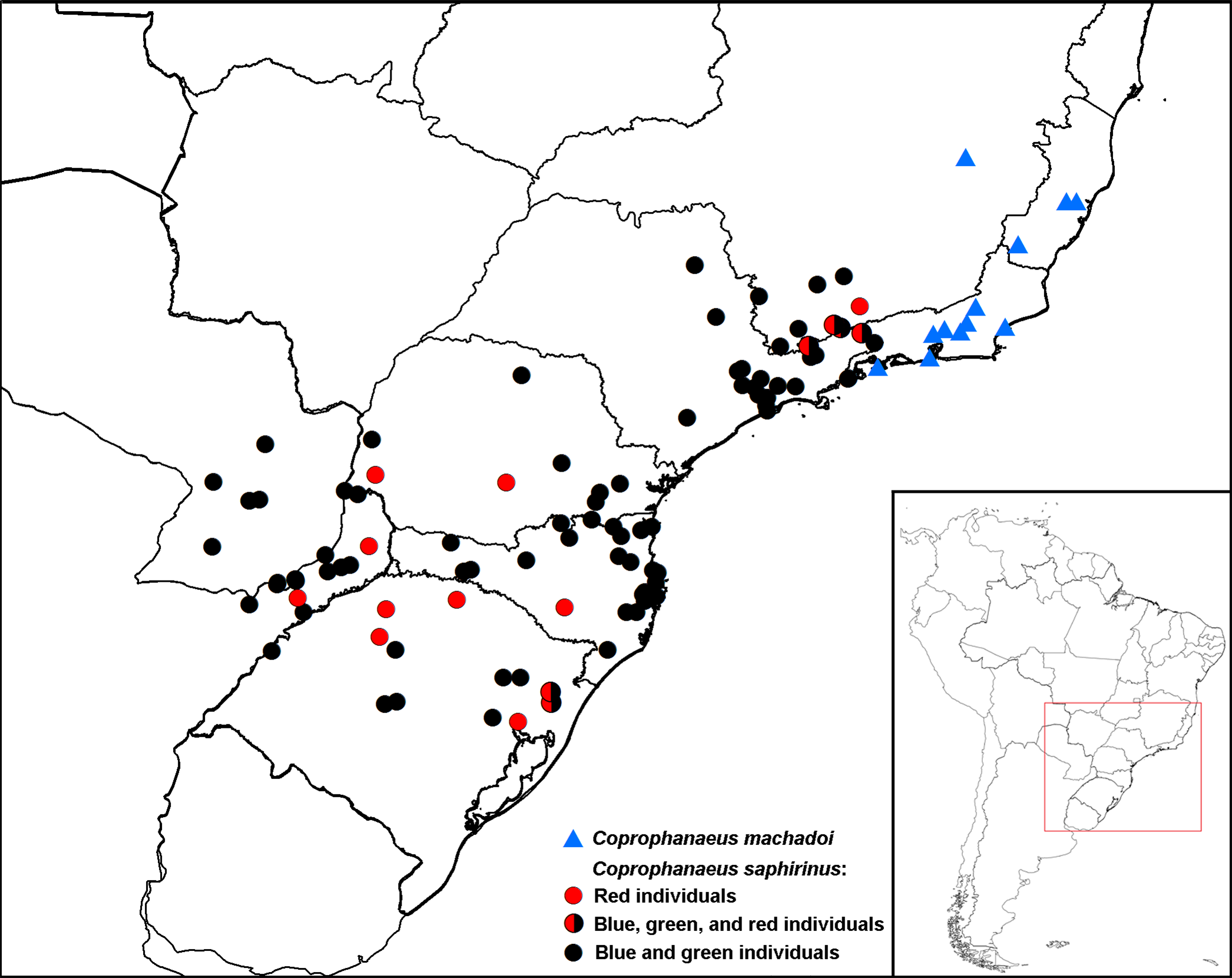
The coloration pattern also has tendencies that differ between the two species. Firstly, a red form has not been observed in C. machadoi , while it is found in C. saphirinus (see the discussion of C. saphirinus ). In C. machadoi , there are both blue and green specimens, some being predominantly light green, while others are dark blue; nonetheless, the most frequent color is dark green. In contrast, in C. saphirinus , dark blue is the most common color, while light green specimens are less frequent. Thus, although it is impossible to differentiate individuals of the two species by color (except red), the relative predominance of blue or green is distinct between C. machadoi and C. saphirinus . Additionally, based on the examined specimens of C. machadoi , it appears that the green color is predominant at lower altitudes, while blue individuals are more common in higher altitudes, but this trend needs confirmation using more specimens.
Finally, biogeography supports the morphological observations. Coprophanaeus machadoi is present from eastern Minas Gerais and Espírito Santo to southern Rio de Janeiro, where it is limited by the Serra da Bocaina mountains, on the border between the states of Rio de Janeiro and São Paulo; south of Serra dos Órgãos (i.e., the northern portion of the Serra do Mar mountains), C. machadoi has been collected only in coastal areas (Rio de Janeiro and Ilha Grande) and is apparently absent in the Vale do Paraíba region in the state of Rio de Janeiro ( Fig. 14 View FIGURE 14 ). In its turn, C. saphirinus is distributed in the Serra da Mantiqueira and in the southern portion of the Serra do Mar (i.e., excluding the Serra dos Órgãos and including the portions localized in São Paulo to Santa Catarina states) south to southern Brazil, Argentina, and Paraguay. Therefore, despite both species being restricted to the Parana dominion of the Chacoan subregion, apparently they are never found in sympatry.
Geographic distribution: Chacoan subregion: Parana dominion: Atlantic and Parana Forest provinces. BRAZIL: Minas Gerais: Açucena. Espírito Santo: Alegre, Fundão (Timbuí), Santa Teresa. Rio de Janeiro: Angra dos Reis ( Ilha Grande), Cachoeiras de Macacu, Cantagalo, Macaé, Nova Friburgo, Petrópolis, Rio de Janeiro (Parque Nacional da Tijuca), Teresópolis (Parque Nacional da Serra dos Órgãos).
Comments: Giving the cited locations, Harold’s (1875) report of Phanaeus saphirinus for Cantagalo (Rio de Janeiro) and the description of the larva of this species by Edmonds (1967) (reared from a female collected in Nova Friburgo, Rio de Janeiro) probably refer to misidentified C. machadoi .
Bionomics: Edmonds (1967) noted that the female of C. machadoi (cited as Phanaeus saphirinus ) was able to build two brood balls even without the assistance of a male; he also observed that the duration of the development from egg to pupa lasted about 90 days. The female was collected using human excrement as bait.
There are collection records of C. machadoi for all months from October to April, suggesting that adults of this species are active only in the months of spring and summer, when the temperature is higher and there is more rain.
Conservation status: Coprophanaeus machadoi is regarded as a critically endangered species in the state of Minas Gerais and is included in its official list of protected species (Minas Gerais, 2010; Biodiversitas, 2007). Nonetheless this species is not present on the Brazilian Red List of endangered species ( Machado et al., 2008) nor is it considered in the IUCN Red List (IUCN, 2013). Although C. machadoi is certainly present in at least two Brazilian National Parks, Parque Nacional da Tijuca and Parque Nacional da Serra dos Órgãos, both in the state of Rio de Janeiro, this species is probably negatively impacted by the continuous deforestation and fragmentation of the Brazilian Atlantic Forest (an impact that was vividly exposed by Ribeiro et al. (2009)), especially because it is well demonstrated that Coprophanaeus species have a strong preference for forest habitats and, in many cases, are restricted to this environment ( Edmonds, 1972; Edmonds & Zidek, 2010).
Material examined: 73 males and 53 females. NO DATA— 1 male ( CEMT), 9 males ( MNHN), 19 males and 12 females ( BMNH), and 14 males and 12 females ( OUMNH). BRAZIL: no more data— 8 males ( MNHN) and 2 males ( BMNH). ESPÍRITO SANTO: no more data— 1 male and 2 females ( MNHN); Alegre, Fazenda Jerusalém, 03.XI.1912, J. F. Zikán col.— 1 female ( FIOC); Santa Teresa, II.1991, O. Roppa col.— 3 females ( MNRJ). MINAS GERAIS: Rio José Pedro, without date, J. F. Zikán col.— 1 male ( CEMT); RIO DE JANEIRO: without date and collector— 5 males and 5 females ( MNHN) and 3 males and 1 female ( MNRJ); Angra dos Reis, Ilha Grande, IV.1992, Theotonio col.— 1 male and 1 female ( CEMT); Cachoeiras de Macacu, Boca do Mato, I-II.2000, N. Tangerini col.— 1 male ( MNRJ); Cantagalo (Cantagallo), without date and collector— 3 males and 1 female ( MNHN); Macaé, Serra de Macaé, XI.1909, Garbe col.— 2 males and 1 female ( MZSP); Nova Friburgo, II.1998, P. Grossi col.— 1 male and 1 female ( CEMT); Nova Friburgo, Macaé de Cima (Haut Macahe), without date and collector— 2 females ( MNHN); Nova Friburgo, Macaé de Cima, 1,500 m., III.2000, Lopes-Andrade, Gumier & Vaz-de-Mello cols.— 1 male ( CEMT); Nova Friburgo, Macaé de Cima, XII.2000, P. & E. Grossi cols.— 1 male and 1 female ( CEMT); Nova Friburgo, Muri, XII.1976, Gred & Guimarães cols.— 1 female ( MZSP); Nova Friburgo, Muri, XII.1980, Gred & Guimarães cols.— 1 male ( MZSP); Petrópolis, II.1939, J. C. N. Penido col.— 1 male ( FIOC); Petrópolis, II.1957, H. Clark col.— 1 male ( BMNH); Petrópolis, Alto Mosele, La Vallon, 01.II-08.III.1957, Dzley col.— 1 female ( MNRJ); Petrópolis, Independência, without date, Mario Rosa col.— 1 male ( MNRJ); Rio de Janeiro, Floresta da Tijuca, 20.XII.1966, Celso Jr. col.— 1 female ( CEMT); Rio de Janeiro, Floresta da Tijuca, XI.1983, C. Godinho col.— 1 male ( CPFA); Rio de Janeiro, Floresta da Tijuca, Represa Rio Grande, X.1962, M. Alvarenga col.— 1 female ( MZSP); Rio de Janeiro, Sumaré, XI.1994, Celso Godinho col.— 1 female ( CEMT) and 1 male ( CPFA); Serra dos Órgãos, XII.1940, Parko col.— 1 male ( MNRJ); Teresópolis, XII.1939, Freitas col.— 1 male ( MNRJ); Teresópolis, VIII.1940, without collector— 1 male ( CPFA); Teresópolis, 14.I.1993, A. Belo col.— 1 male and 3 females ( CEMT); Teresópolis, Parque Nacional da Serra dos Órgãos, 26.I.1957, D. Zajciw col.— 1 female ( MNRJ); Teresópolis, Parque Nacional da Serra dos Órgãos, 25.X.1960, D. Zajciw col.— 1 female ( MNRJ). URUGUAY (surely mislabeled): Montevideo, without date and collector— 1 male ( BMNH).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Scarabaeinae |
|
Tribe |
Phanaeini |
|
Genus |
|
|
SubGenus |
Metallophanaeus |
Coprophanaeus (Metallophanaeus) machadoi ( Pereira & d’Andretta, 1955 )
| Cupello, Mario & Vaz-De-Mello, Fernando Z. 2014 |
Coprophanaeus (Metallophanaeus) machadoi
| Edmonds 2010: 2 |
| Biodiversitas 2007: 123 |
| Arnaud 2002: 56 |
Phanaeus (Metallophanaeus) saphirinus : sensu
| Edmonds 1967: 97 |
Phanaeus (Metallophanaeus) machadoi Pereira & d’Andretta, 1955 : 257
| Edmonds 2010: 29 |
| Pereira 1955: 257 |
Phanaeus saphirinus : sensu
| Harold 1875: 42 |