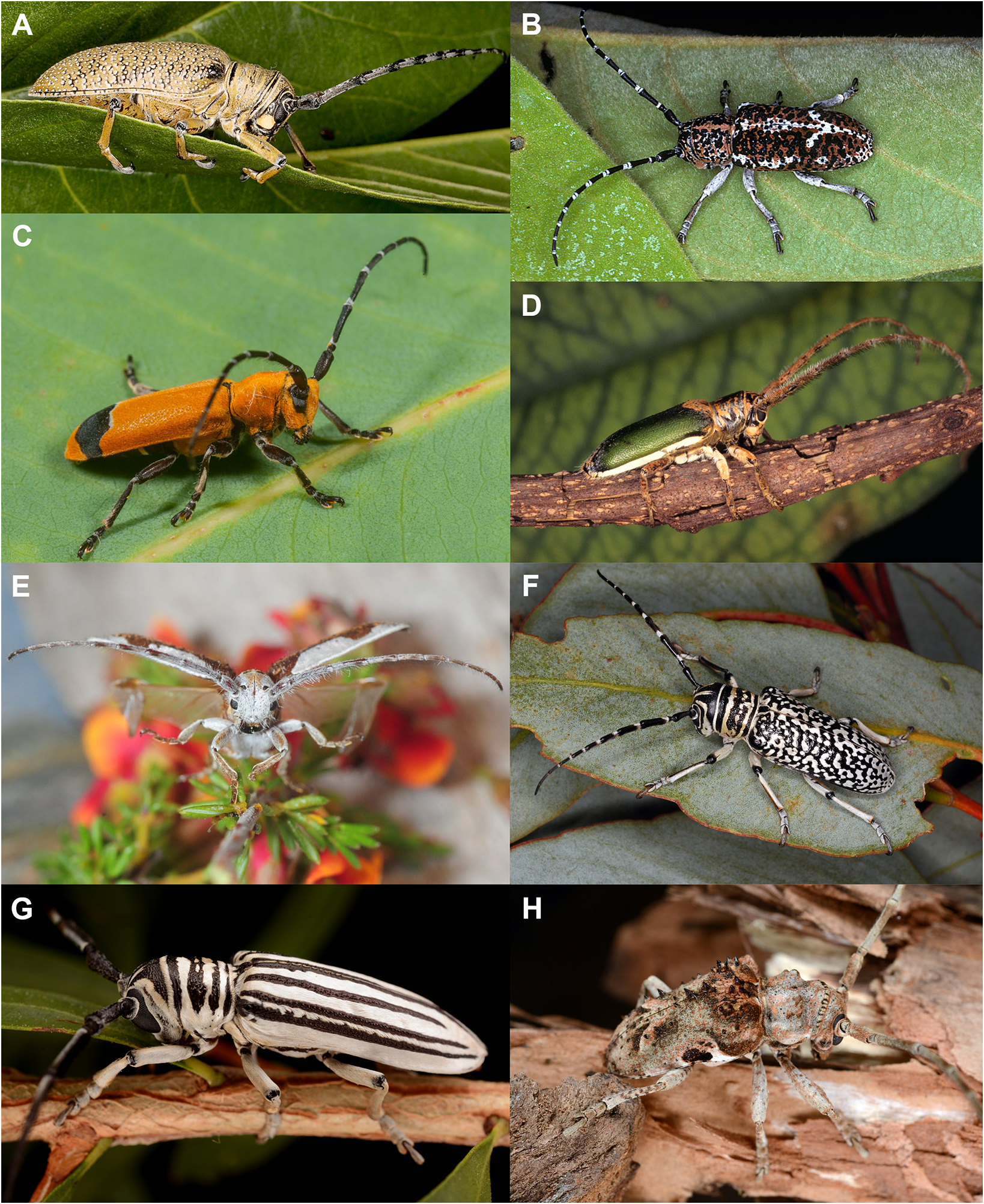
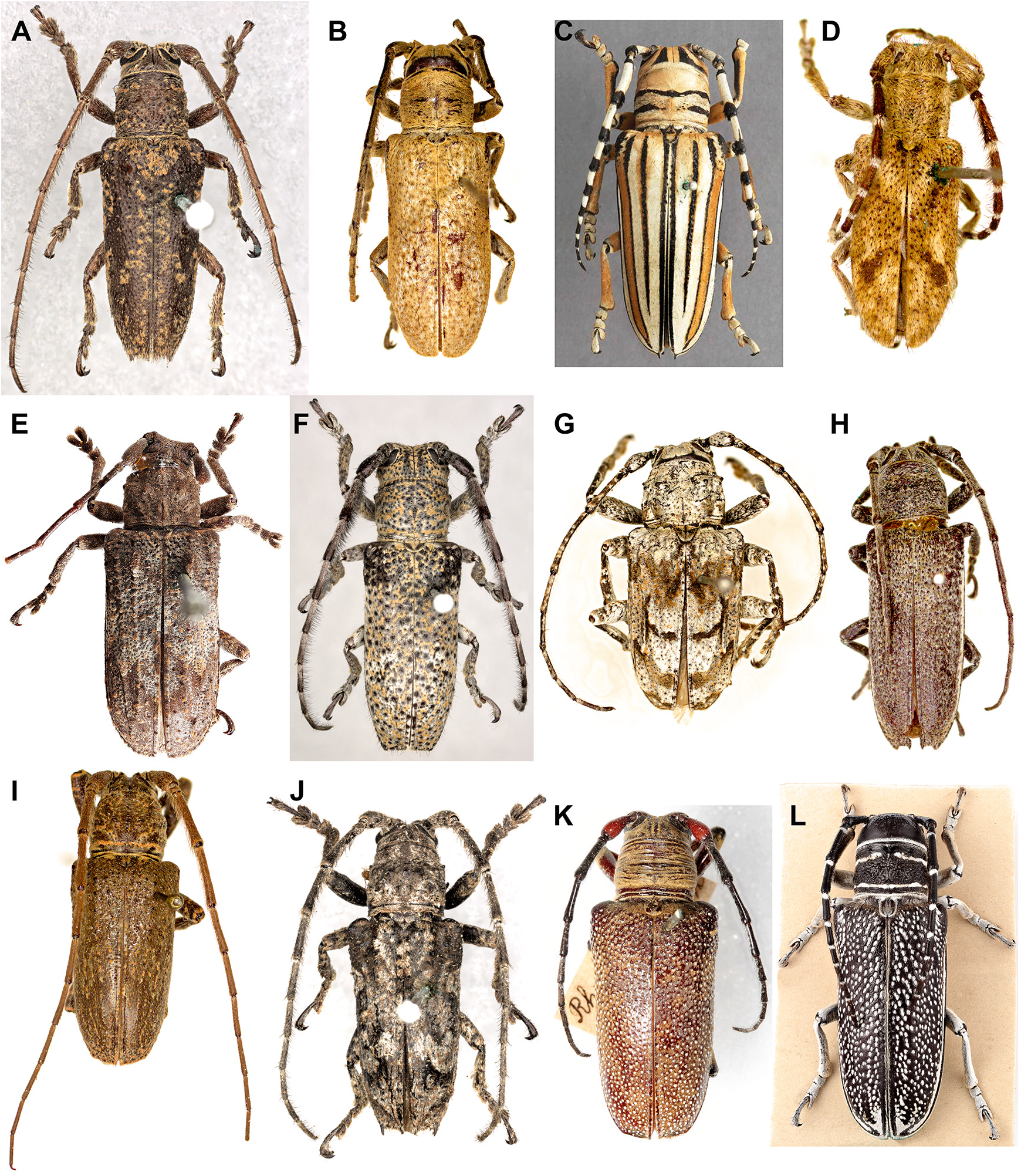
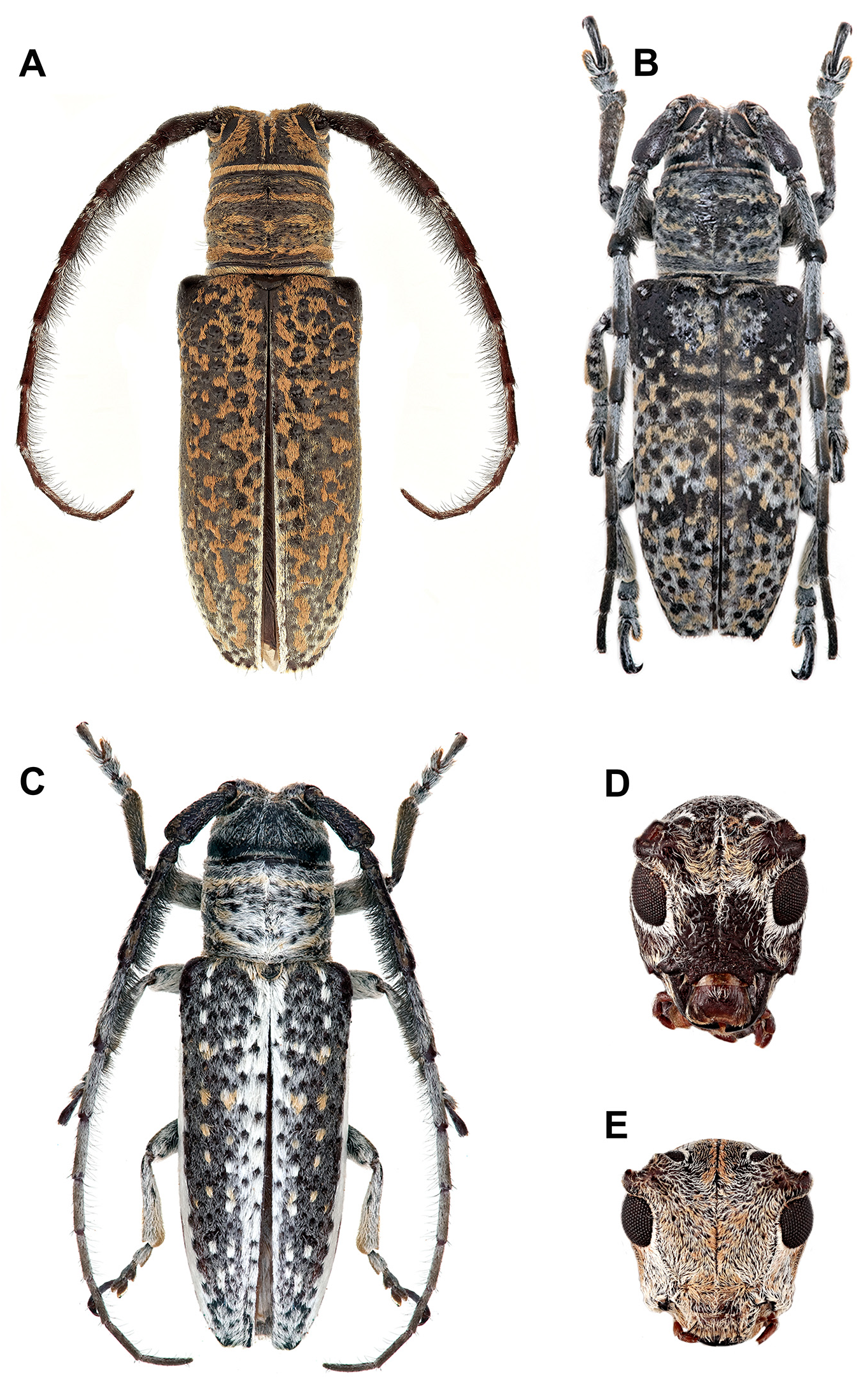
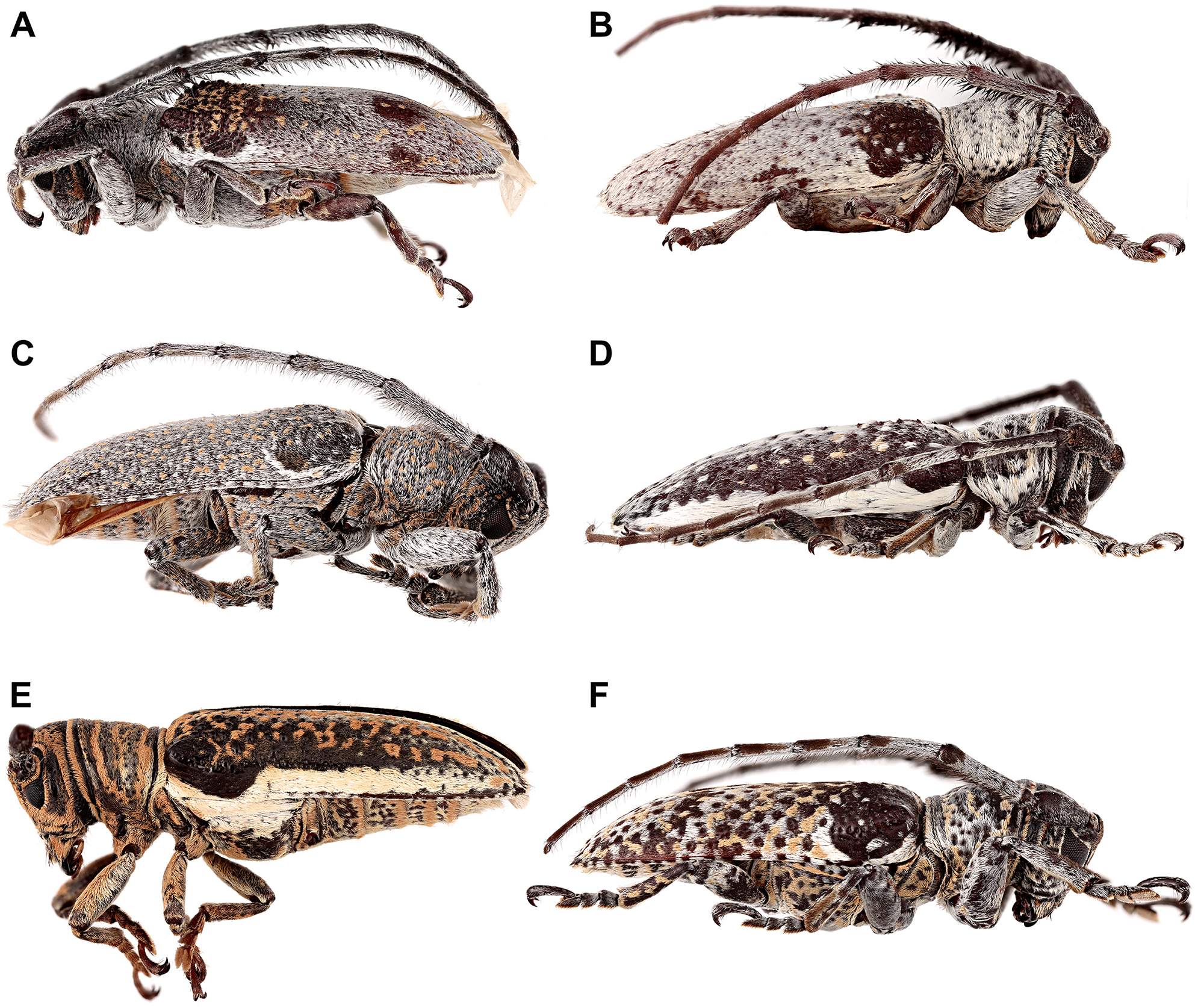
Rhytiphora, Audinet-Serville, 1835
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5312.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:E45A10FC-CB08-4C66-B1E9-B6857C58343B |
|
DOI |
https://doi.org/10.5281/zenodo.8146841 |
|
persistent identifier |
https://treatment.plazi.org/id/F8183D32-037F-7F59-FF2B-861B9C3AFD23 |
|
treatment provided by |
Plazi |
|
scientific name |
Rhytiphora |
| status |
|
Rhytiphora View in CoL View at ENA species complexes
Rhytiphora argus Pascoe, 1867 , R. dispar Blackburn, 1894 , R. nigropunctata Breuning, 1938 and R. nigrosparsa Breuning, 1938 are all described from QLD and are fairly similar morphologically (large-bodied and large-eyed with black spots on the elytra). These four species need to be sequenced to confirm whether they are distinct genetic lineages, or one species with variable setae colouring (yellow/orange to grey).
The species R. bankii Fabricius, 1775 is extremely widespread, with records from Australia through Southeast Asia to China, Japan and even Hawaii (although this may be a human introduction; McKeown 1947). Indeed, the type locality in the original publication is listed as South Africa (Cape of Good Hope; Fabricius 1775), though this may be incorrect; Gahan (1893) does not discuss his reasoning for synonymising Lamia bankii with Acanthocinus hollandicus and other Australasian species. Given that no other Rhytiphora species has such an extensive distribution, R. bankii specimens from across the Pacific region should be sequenced to determine whether there are cryptic species; we already have mitochondrial data which show that two specimens from Queensland and Christmas Island (south of Java, Indonesia) have genetic divergence equivalent to that of other Rhytiphora species ( Ashman et al. 2022a).
Rhytiphora cana McKeown, 1948 , R. cinerascens Aurivillius, 1917 , R. deserti Blackburn, 1896 and R. satelles Pascoe, 1865 are very similar morphologically and need sequencing to confirm the species boundaries. There is at least one specimen (ANIC 25-66662) from northern NT which is morphologically closest to R. deserti (central Australia) but genetically closer to R. cinerascens (QLD; Ashman et al. 2022a).
Rhytiphora cinnamomea Pascoe, 1859 (QLD), R. gallus Pascoe, 1864 (central Australia) and R. ferruginea Aurivillius, 1917 (WA) have very similar colouration but different eye sizes ( R. cinnamomea lower lobes separated by 3.2x eye width; R. gallus 2.4x; R. ferruginea unknown). Phylogenetically they form a clade with R. fasciata Blackburn, 1901 and R. tuberculigera Breuning, 1938 ( Ashman et al. 2022a), which are both described from QLD and morphologically distinct. The R. cinnamomea specimen (ANIC 25-66541) is more closely related to a specimen from WA with similarly small eyes (ANIC 25-66555) than it is to another QLD specimen with larger eyes (ANIC 25-66546). More extensive sampling, combined with an examination of the R. ferruginea holotype, is needed to determine how many lineages there are and which described species, if any, they correspond to.
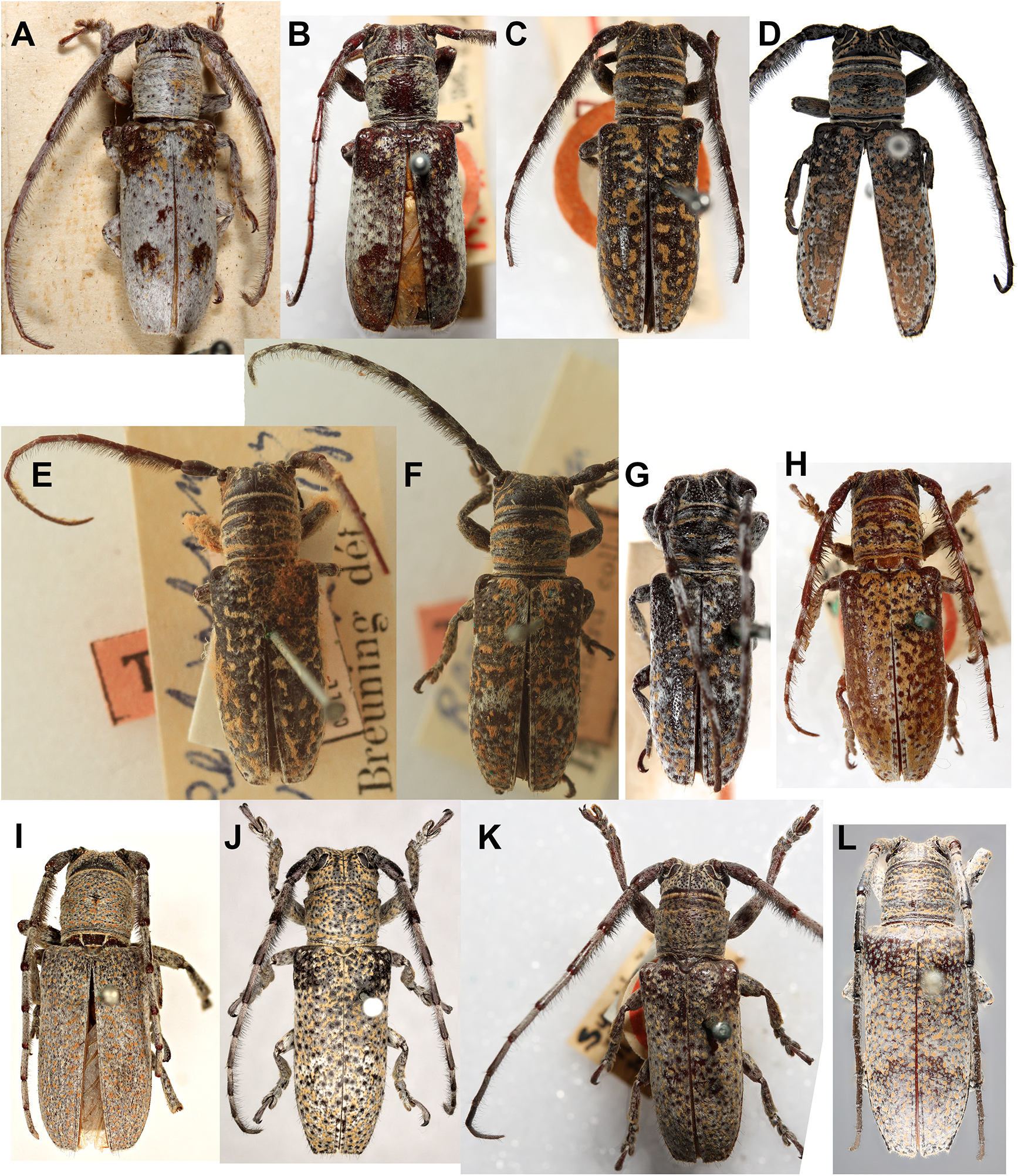
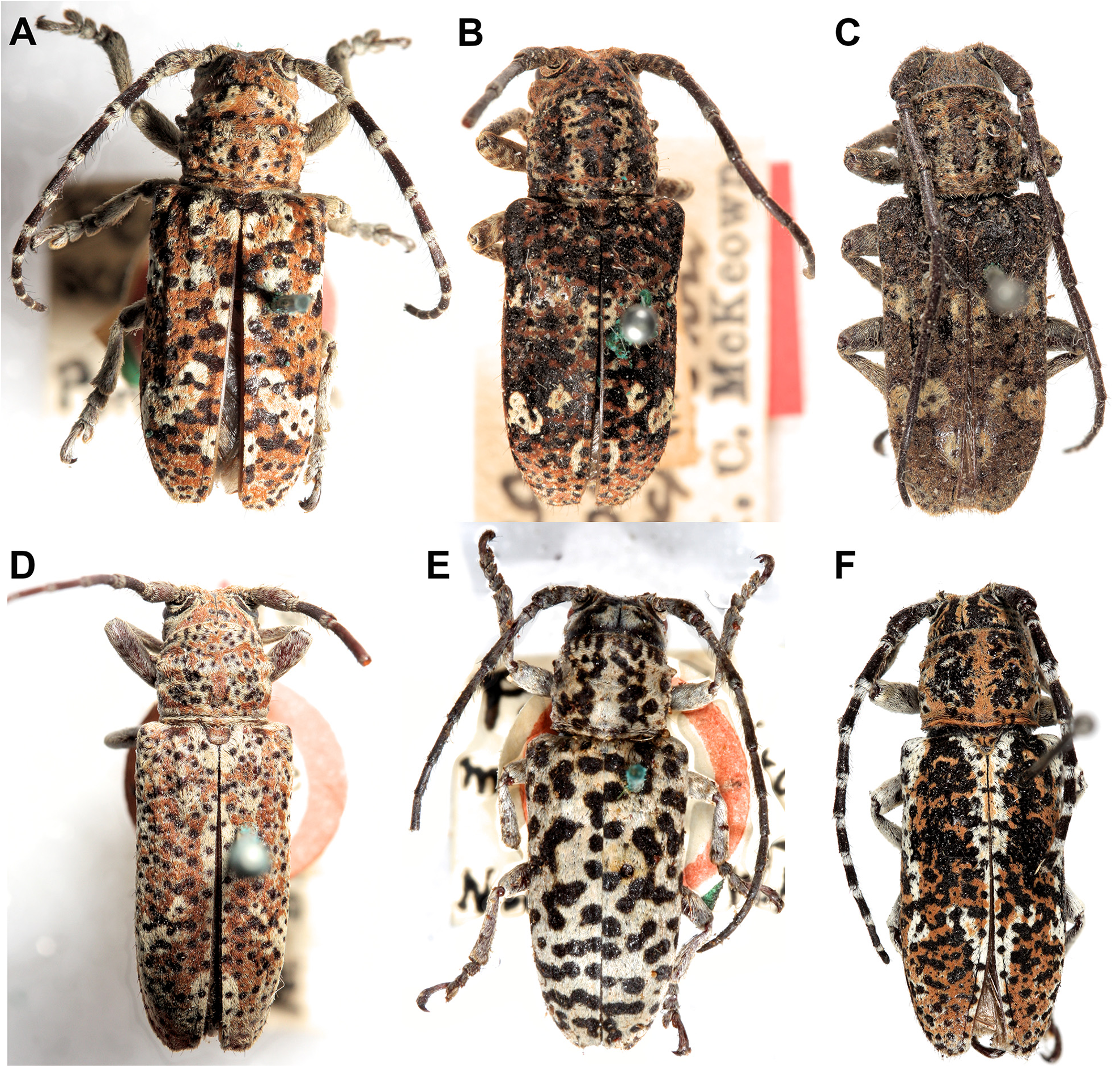
We synonymised several morphologically similar species under R. collaris ( Figs. 7A View FIGURE 7 , 8E View FIGURE 8 ): Saperda albocincta , R. intertincta , R. parafarinosa , R. vermiculosa and Symphyletes vestigialis ( Figs. 10C–H View FIGURE 10 ). We used the oldest available name for this species, although the holotype of Saperda collaris is missing and the species is depicted as having entirely black elytra ( Donovan 1805). In his description of Saperda albocincta, Guérin-Méneville (1831) notes that the holotype of S. collaris is very similar to his new species and may have simply lost the yellow setae that form the distinctive elytral patterning seen in S. albocincta . In the absence of any specimens with black elytra matching S. collaris , and on the basis of Guérin-Méneville’s comment, we have synonymised these species. A neotype has been designated for Saperda collaris using the only specimen with published genetic data: ANIC 25- 066530 ( Ashman et al. 2022a; Fig. 10D View FIGURE 10 ). We also synonymised the subspecies of S. albocincta as they did not make geographic sense (grouping NSW with SA rather than QLD); the corresponding morphological trait (full or patchy white lateral stripe on elytra) is noted beside each type in the checklist.
Rhytiphora costata Pascoe, 1863 (Australia-wide), R. intricata Pascoe, 1864 (SA), R. tigrina Blackburn, 1901 (WA) and R. vermicularia Donovan, 1805 (east coast) have different colour patterns but very similar body size, shape and sculpturing (large-bodied, oval, transverse rugose pronotum, longitudinally ridged elytra). Sequencing is needed to confirm species distributions and boundaries (i.e. R. tigrina may be a yellow western morphotype of R. vermicularia ); it is likely these species form a clade with the distinctive R. pardalis Newman, 1842 which, like R. costata , is widely distributed across Australia.
The holotypes of R. cretata Pascoe, 1859 (QLD) and R. heros Pascoe, 1863 (NT) have similar elytral patterns but different antennae and pronotum colouring (plain vs. striped). There are also intermediate specimens from QLD with plain antennae and striped pronotum (e.g. Fig. 134D in Ślipiński & Escalona 2013). It is possible that R. heros is the same species as R. cretata , and the holotype has simply lost the setae on its antennae and pronotum; sequencing specimens across the geographic and morphological gradients will provide further insight.
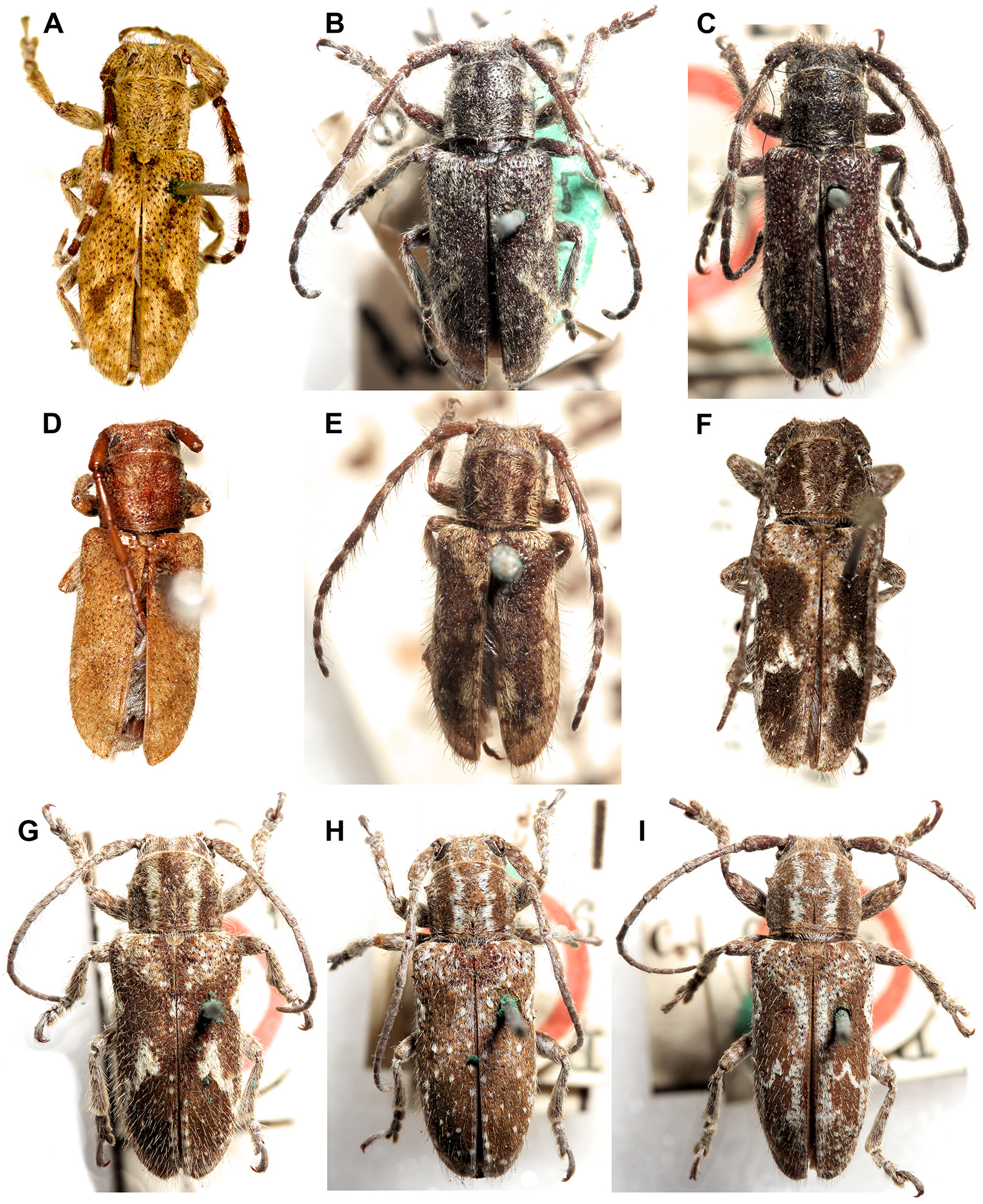
Rhytiphora detrita Hope, 1842 (north and west coast; Fig. 2A View FIGURE 2 ) and R. variolosa Pascoe, 1862 (east coast) are quite similar morphologically but do have different elytral patterns (ochre lateral stripe and dorsal mottling vs. just ochre spots). However, Ashman et al. (2022a) found very little genetic divergence between these two species; more extensive sampling is needed to determine whether R. detrita and R. variolosa are separate species or one widespread species.
We examined the holotypes of Corrhenes flavovittata Breuning, 1938 and C. flavovittata demarzi Breuning, 1963 and found several morphological differences (e.g. antennae and elytral setae patterns; Fig. 11 View FIGURE 11 ). We therefore elevated C. flavovittata demarzi to species status (here renamed R. rentzi new name), pending genetic confirmation: R. rentzi is quite similar to R. stigmatica Pascoe, 1863 . We have synonymised C. flavovittata with R. paulla Germar, 1848 , along with several other species with similar white markings on the antennae, pronotum and elytra: Saperda funesta Pascoe, 1859 , Anaesthetis lepida Germar, 1848 and Cobria rufa Breuning, 1961 . We have used the name R. paulla as that holotype is presumably better preserved than A. lepida , which from its description (in the same publication as R. paulla ) seems to have lost most of its setae. It would be worthwhile using sequence data to determine how many lineages exist in this large species complex of R. paulla .
Rhytiphora fraserensis Blackburn, 1893 and R. obenbergeri Breuning, 1938 ( Figs.3B,3F View FIGURE 3 ) overlap geographically and have only subtle differences in morphology (eye size, elytral setae); sequencing is needed to confirm whether they are separate species. The widespread R. lateralis Pascoe, 1858 also has similar elytral colouring but can be distinguished by the waviform pattern of the brown dorsal and white lateral setae, as well as the ochre stripe on the pronotum.
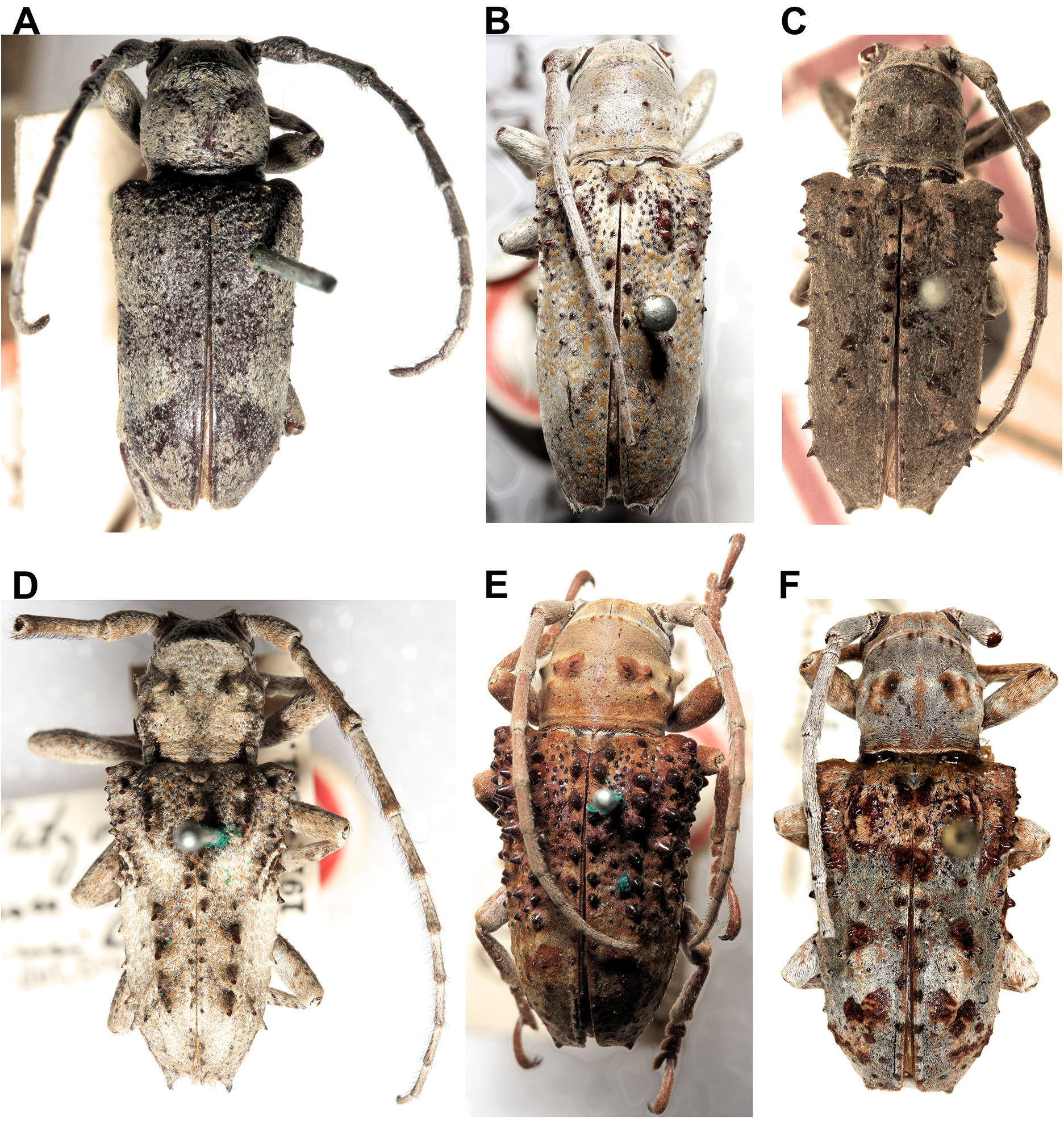
Rhytiphora frenchi Blackburn, 1890 and R. frenchiana Breuning, 1961 have similar names but are very different species. Most of the specimens we have seen labelled as R. frenchi in Australian collections match the description of Blackburn’s (1895) Rhytiphora frenchi , now renamed R. frenchiana by Breuning ( Fig. 2L View FIGURE 2 ): large-bodied (18 lines = ~ 38 mm) with a mottled black and white pattern similar to that of R. saundersii Pascoe, 1857 ( Fig. 1F View FIGURE 1 ; also see below). The senior homonym, R. frenchi (originally Platyomopsis ; Blackburn 1890), is a smaller grey-haired species resembling R. armatula White, 1859 and R. obliqua Donovan, 1805 ( Fig. 1H View FIGURE 1 ) but without such pronounced elytral spines. These three species, as well as R. multispinis Breuning, 1938 , R. regularis Gahan, 1893 and R. subregularis Breuning, 1973 ( Fig. 12 View FIGURE 12 ), are often confused and would benefit from sequencing to confirm the species boundaries (especially for R. regularis and R. subregularis , which may be red western/brown eastern morphotypes of the same widespread species).
Rhytiphora freyi Breuning, 1961 ( Fig. 4D View FIGURE 4 ) has similar elytra to R. villosa Breuning, 1938 (colour pattern and basal tufts of long setae) but a different pronotum (colour pattern more similar to R. oblita Pascoe, 1863 ). Sequencing is needed to determine how many lineages exist.
Rhytiphora fulvescens and R. subtuberculata White, 1858 have similar body shape and white markings on the elytra, but are predominately different colours. White’s (1858) description of Symphyletes subtuberculatus mentions an ochreous variant of the grey holotype, which may refer to what Pascoe (1863) described as Symphyletes fulvescens . Sequencing of both colour varieties is needed to confirm whether there are one or two species.
Rhytiphora fumata Pascoe, 1864 and R. obscura Breuning, 1938 are quite similar morphologically, but R. obscura has much fainter elytral patterning. Closer examination of the R. obscura holotype, and genetic sequencing of similar specimens (if not the original holotypes themselves), will determine whether these two species should be synonymised.
The colour pattern of R. glauerti McKeown, 1948 appears to be intermediate between that of R. crassicollis (banded pronotum) and R. macularia Pascoe, 1867 (finely mottled elytra; Fig. 4I View FIGURE 4 ). Only R. crassicollis has been sequenced ( Ashman et al. 2022a); more extensive sampling, across all three species’ geographic ranges, is needed to confirm the species boundaries.
Rhytiphora maculicornis Pascoe, 1858 and R. sospitalis Pascoe, 1865 (both WA) are very similar morphologically except for a white diagonal marking in the apical third of the elytra (present in R. maculicornis , absent in R. sospitalis ). There are also two species from QLD with similar body size, shape and colouring: R. parantennalis Breuning, 1970 and R. ochreomarmorata Breuning, 1939 . Sequencing of all four species will determine how many lineages there are.
Rhytiphora marmoreoides Tavakilian & Nearns, 2014 (WA), R. rosei Olliff, 1890 (east coast) and R. saundersii (western to central Australia) share a distinctive black and white elytral pattern ( Fig. 1F View FIGURE 1 ); R. marmoreoides and R. rosei are more finely mottled than R. saundersii , yet do not overlap geographically. Sequencing specimens from across Australia is needed to confirm the species boundaries. Rhytiphora frenchiana also has black and white elytral patterning, but can be distinguished from the above three species by the white lateral stripe on the elytra ( Fig. 2L View FIGURE 2 ).
Rhytiphora mastersi Blackburn, 1897 (WA), R. melanosticta Pascoe, 1875 (WA and NT) and R. scenica Pascoe, 1863 (QLD) have similar morphology (banded antennae, orange/white elytra with black spots; Fig. 13 View FIGURE 13 ). There are several specimens with colouration partway between R. mastersi and R. melanosticta ; sequencing is required to determine the species’ genetic, morphological and geographic boundaries. Rhytiphora pardalina Breuning, 1942 ( Figs. 1B View FIGURE 1 , 13F View FIGURE 13 ) has a similar colour scheme to the above three species, but lacks a lateral pronotum spine and has a unique elytral pattern consistent across many specimens.
Rhytiphora ochreopicta Breuning, 1940 is similar to R. oblita except for the white diagonal marking in the apical third of the elytra (absent in R. ochreopicta , present in R. oblita ). Sequencing is needed to establish whether or not this morphological difference corresponds to distinct lineages.
There is a complicated history of R. piligera and R. pulverulens Boisduval, 1835 , with some species incorrectly synonymised with the former instead of the latter due to mislabelling of specimens in various museums ( McKeown 1947). Here we have restricted R. piligera to the original type, housed in ANIC ( Fig. 2E View FIGURE 2 ), and the newly synonymised Symphyletes nodosus Newman, 1842 (which are both brown with basal elytral tubercules and clavate antennal scape). We united all the large, grey, diagonally striped species under R. pulverulens : Saperdopsis armata Thomson, 1864 , Symphyletes anaglyptus Pascoe, 1867 , S. ingestus Pascoe, 1863 , S. moratus Pascoe, 1863 , S. munitus Pascoe, 1863 , S. sodalis Pascoe, 1859 and S. vetustus Pascoe, 1862 (but not R. devota Pascoe, 1866 from WA). Defined thus, this species is distributed widely across the eastern half of Australia, as well as New Guinea; sequencing specimens across this range would allow us to determine whether R. pulverulens is one widespread or multiple convergent species. Rhytiphora sundaensis Breuning, 1973 ( Fig. 2I View FIGURE 2 ) from the Maluku province of Indonesia is very similar morphologically to the Australian R. pulverulens , but has been left separate pending genetic confirmation.
The holotypes of R. sannio Newman, 1838 (east coast) and R. waterhousei Pascoe, 1864 (south coast) have very similar body size and shape, but different elytral colouration (red with diagonal markings vs. yellow without markings). However, there are specimens from NSW with intermediate colouration. It is possible that R. waterhousei is a southern variant of R. sannio ; sequencing across the geographic and morphological gradient is needed to determine whether distinct lineages exist.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.