Massonia amoena Mart.
|
publication ID |
https://doi.org/ 10.11646/phytotaxa.181.3.1 |
|
DOI |
https://doi.org/10.5281/zenodo.5152341 |
|
persistent identifier |
https://treatment.plazi.org/id/F6679B3B-C11B-FF98-F88E-FF0FEA88FE73 |
|
treatment provided by |
Felipe |
|
scientific name |
Massonia amoena Mart. |
| status |
sp. nov. |
Massonia amoena Mart. View in CoL -Azorín, M.Pinter & Wetschnig, sp. nov. ( Figs. 2–9 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 View FIGURE 7 View FIGURE 8 View FIGURE 9 )
Species notabilis combinatione unica characterum ab omnibus speciebus Massoniae differt foliis glauco-viridulis, supra numerosas pustulas viridulas, purpurascentes atque rubescentes obsitis, quae valde heterogeneis sunt (aliquas minutas et circulares, ceteras oblongas et longitudinaliter dispositas); segmentis perigonii per anthesin valde reflexis a basi vix sigmoideis; filamentis in tubi breve 1−2.5 mm supra perigonium connatis; antheris et polline omnibus cyanellis; atque ovario in stylo gradualiter desinente.
Type:— SOUTH AFRICA. Eastern Cape: Northern slopes Andriesberg , Alt. 5500 ft. [approx. 1680 m], May 1899, Fl. White, E. E. Galpin 2612 (holotype GRA!, Fig. 2 View FIGURE 2 ) .
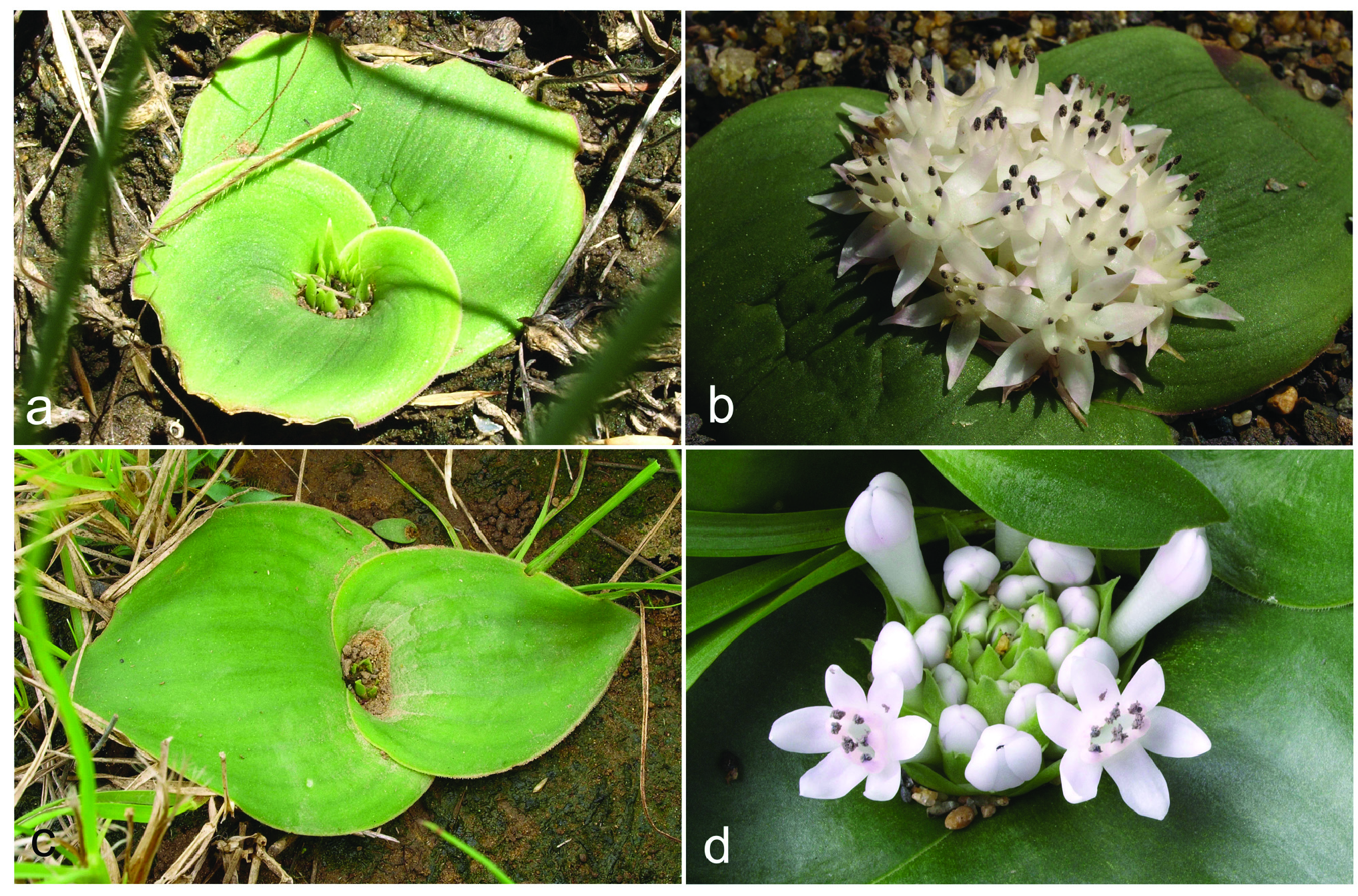
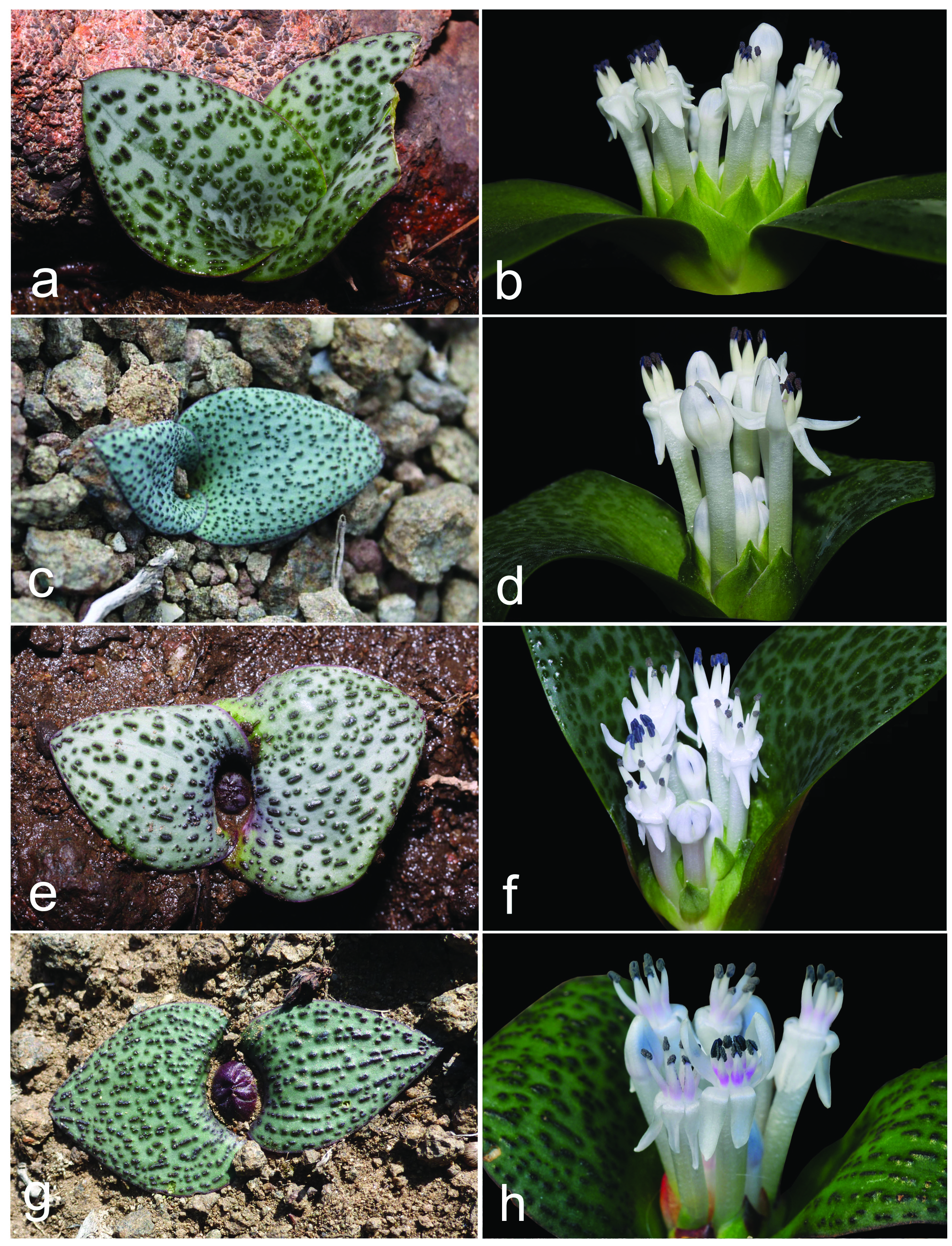
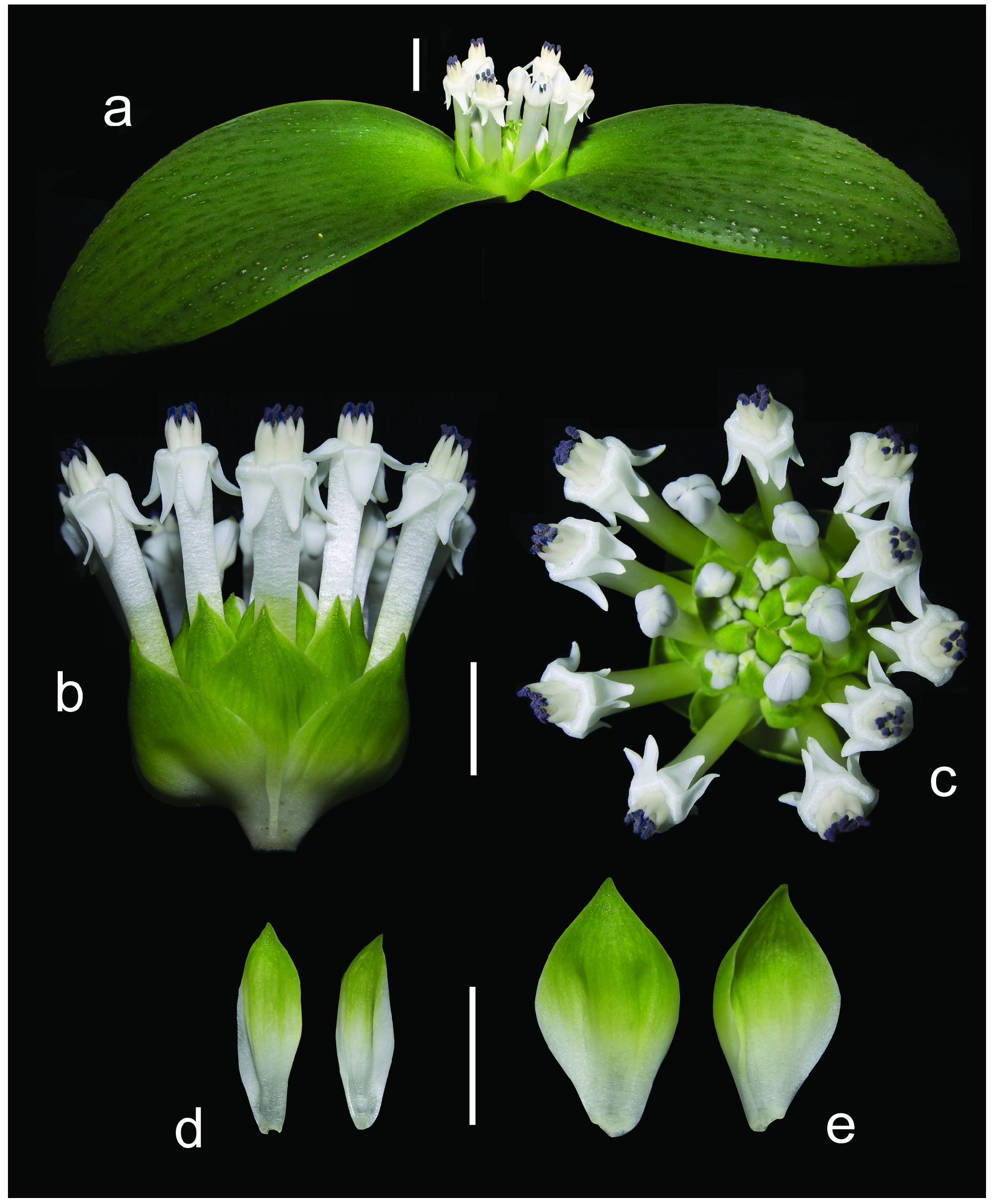
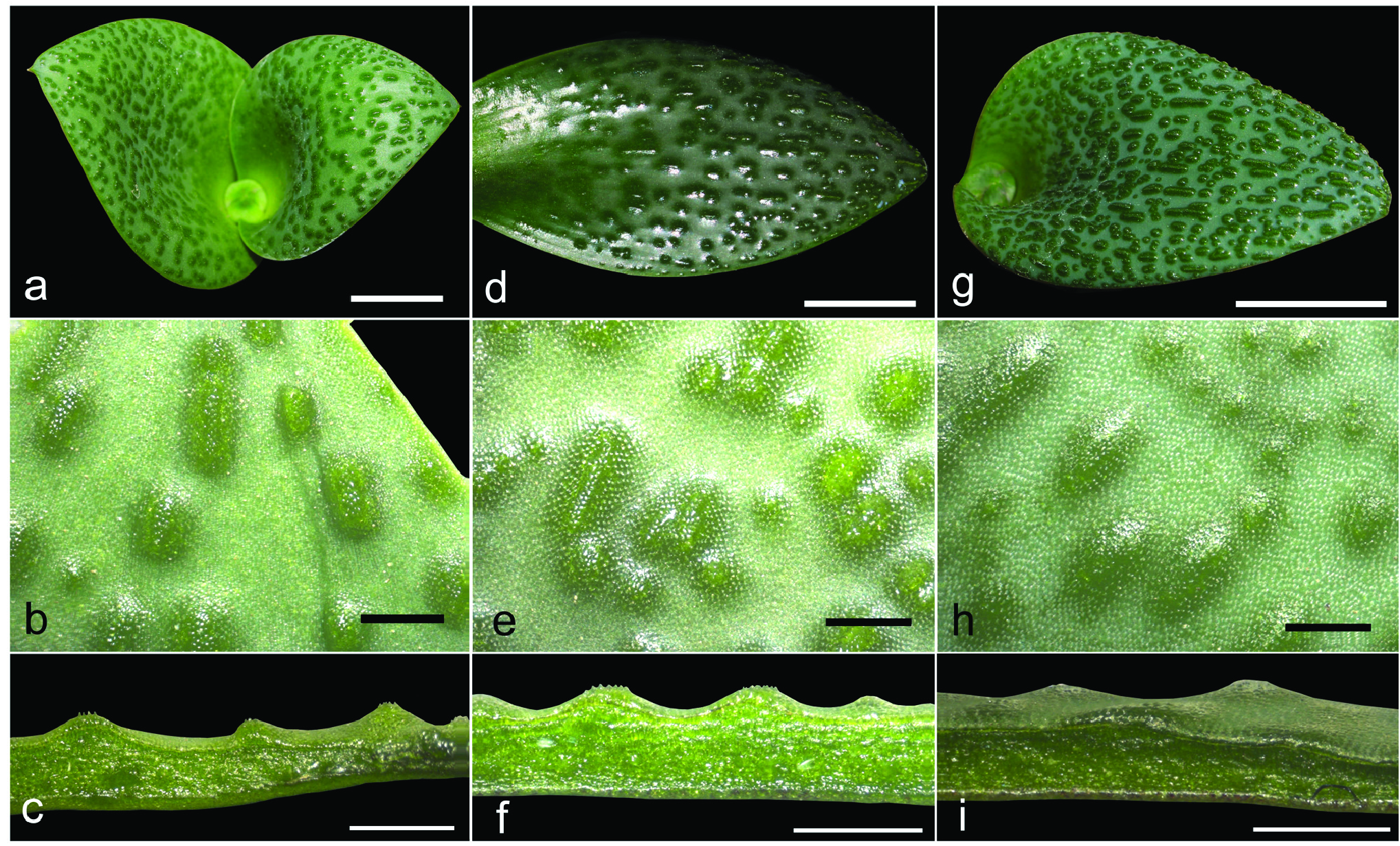
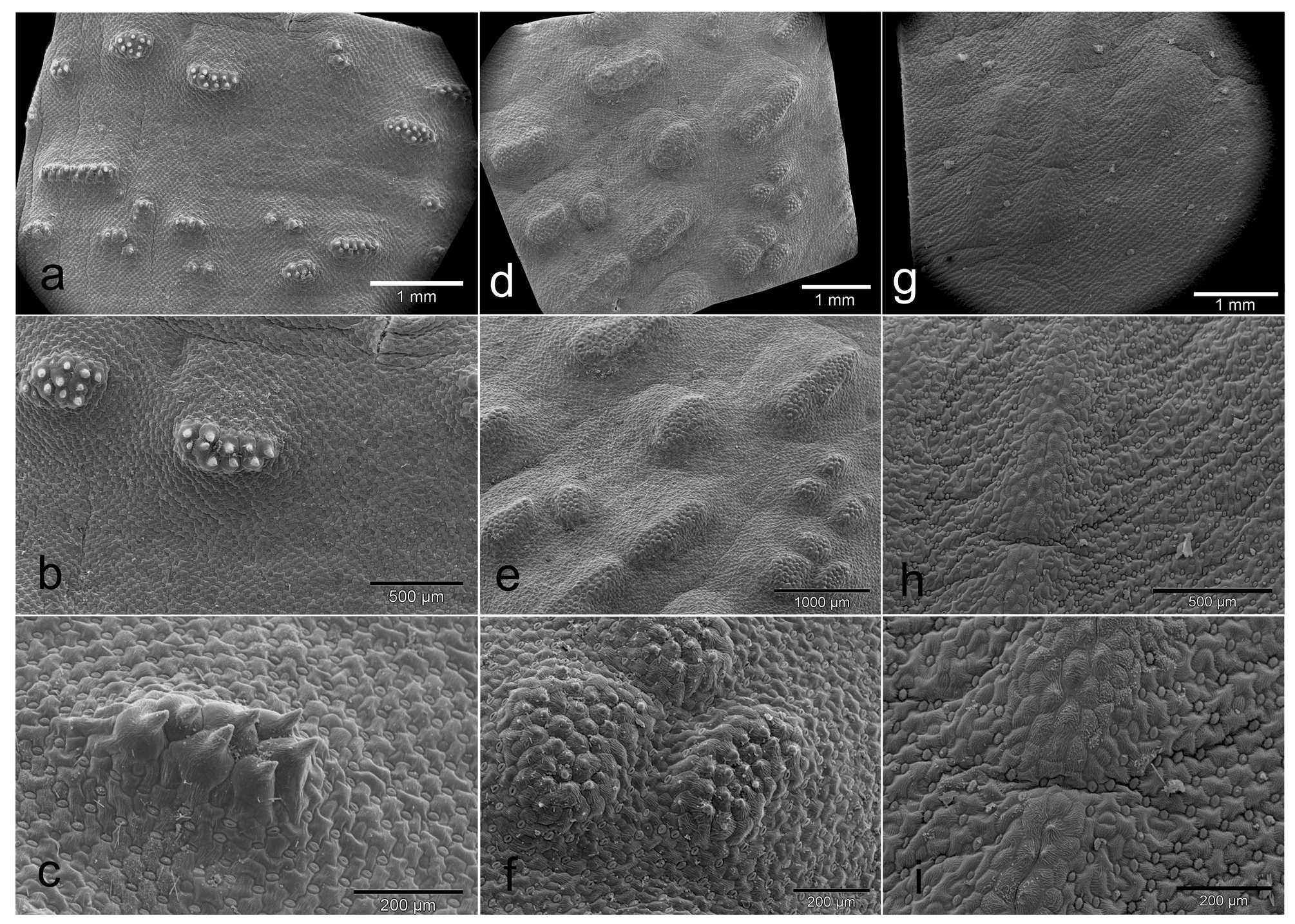
Herbaceous perennial bulbous geophyte. Roots branched, usually present for about two vegetation periods. Bulb ovoid, ca. 15−20 × 10−15 mm, inner scales fleshy and white, outer tunics papery and brownish. Leaves 2, deciduous, leaf blades opposite, spreading and appressed to the ground, 2−8 × 1−5 cm, synanthous, ovate with acute apex, with a short apicule ca. 1 mm long, with minutely papillose or shortly ciliate margin, narrowed into a subterraneous petiole 1−3 cm long that clasp the inflorescence and the peduncle; adaxial side glaucous green with numerous (50−150 per cm 2), dark green, purplish or reddish emergences, being heterogeneous in morphology, from circular (0.3−0.8 mm diameter) to elongated and disposed longitudinally (1−3 × 0.4−0.8 mm), 0.2−0.4 mm high with several short papillae on top; abaxial side green, smooth. Inflorescence a dense, subcapitate raceme, up to 1−2 cm long, with 8−32 flowers, shortly overtopping ground level, but flowers usually long exerted. Bracts membranous, green, sometimes with a purplish flush in the upper half and white below, glabrous with entire margins; lower bracts ovate 14−21 × 10−13 mm; upper bracts narrowly ovate, 12−16 × 5–8 mm. Pedicels 4−8 mm long. Flowers proterandrous, tubular, actinomorphic, with a strong pleasant smell. Perigone white or rarely pinkish, free segments deltoid or narrowly triangular, 4–7 × 1.5–3 mm, white, first straight and erect, later spreading and finally strongly reflexed at anthesis with a very slight curve at the base. Perigone-filaments tube 15−24 × 2.5−3.5 mm at anthesis, cylindrical, white or pinkish. Filaments white or tinged with pink, free portions narrowly triangular, 1–4 mm long, rather fleshy and thickened, straight, erect, connate at the base for ca. 1−2.5 mm above the perigone to form a cylindrical, filaments tube. Anthers ca. 2 mm long when closed, oblong, with dark blue anther wall, dorsifixed. Pollen dark blue. Gynoecium cenocarpous-syncarpous, narrowly obclavate, with septal nectaries; nectar colourless, sometimes yellow when aged. Ovary oblong, green, 3.8– 4.8 × 1.8–2.2 mm, with 10–14 ovules/locule; style white, 9–17 × 1–1.4 mm, thick, narrowly triangular, erect, gradually tapering to the punctiform stigma, shorter or as long as the perigone-filaments tube. Capsule loculicidal, 10–14 × 5–8 mm, valves splitting down to the base, ovate-oblong in lateral view and trigonous in apical view. Seeds black, with a greyish overlay (epicuticular wax), somewhat glossy, 2–2.4 × 1.6–2 mm, ellipsoidal, flattened at the chalazal region, with an inclined, conical apex at the micropylar region. ( Figs. 3–9 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 View FIGURE 7 View FIGURE 8 View FIGURE 9 ).
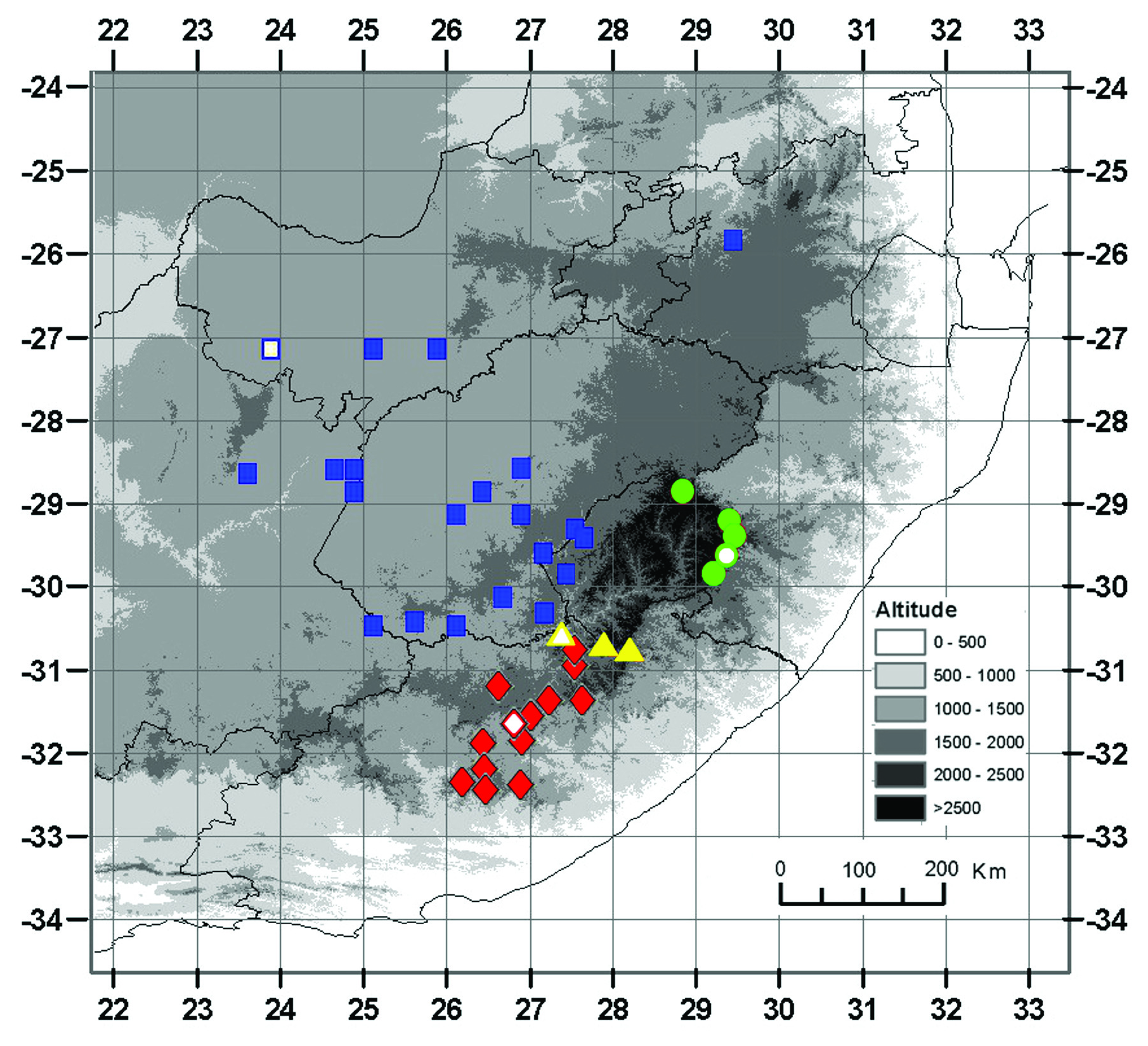
Taxonomic relationships: — Massonia amoena can be easily distinguished from all other species in the genus by the glaucous green adaxial side of the leaves with numerous, dark green, purplish or reddish, morphologically heterogeneous emergences, these being from small and circular to elongated and disposed longitudinally; the strongly reflexed perigone segments at anthesis with a slight sigmoid curve at the base; the filaments connate for 1−2.5 mm above the perigone; the blue anthers with blue pollen; and the style gradually tapering from the ovary ( Figs. 3–9 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 View FIGURE 7 View FIGURE 8 View FIGURE 9 ). Its closest known relative appears to be Massonia jasminiflora , which shares the long perigone-filaments tube, the connate filaments, the blue anthers and pollen and the pleasant sweet smell. However, M. jasminiflora differs in the smooth leaves, commonly ciliate, the patent free portions of the perigone segments at anthesis, and its allopatric distribution ( Fig. 1 View FIGURE 1 , 10 View FIGURE 10 ; Table 2). Massonia wittebergensis and M. saniensis share with M. amoena the leaves bearing emergences, the reflexed perigone segments, the connate filaments and blue anthers, but both species differ by the shorter perigonefilaments tube, the yellow pollen, the very different morphology of leaf emergences and their allopatric distribution ( Table 2, Fig. 10 View FIGURE 10 ).
Etymology:—Species named after the striking and beautiful leaves and flowers (amoenus,– a,– um: pleasant, delightful).
Biology: — Massonia amoena starts to emerge from dormancy during late summer in its natural habitat. Plants grow mostly from late February to early April and by the end of April (autumn in the southern hemisphere) leaves have reached their maximum development. Flower buds begin to form as early as February but they do not start to develop properly until April ( Craib & Knoll 2000). Massonia amoena flowers mainly in May and June in the wild. In cultivation in Europe they flower from late October to December. Craib & Knoll (2000) commented that honey bees and a variety of flies were observed visiting flowers of cultivated plants, suggesting that there may be more than one pollinating agent in wild populations. The very intense pleasant smell, the long perigone-filaments tube and the white flower colour would suggest pollination by moths. A more detailed study based on wild populations is necessary to evaluate these statements.
Habitat: —The new species occurs at elevations from approximately 1300 to 2300 m, mainly in seasonally moist seepage areas facing south, west or east. It is usually found on rocky slopes at the base of large sheets of exposed dolerites, basalt or sandstone, but also in flat open grassveld with rocky patches. These habitats experience cold weather with severe frost in May and June at the peak of Massonia amoena flowering season. Temperatures are often well below freezing and snow may fall on the mountains ( Craib & Knoll 2000). All known populations of Massonia amoena are confined to the Grassland Biome sensu Mucina & Rutherford=2006 (). The northern populations from the Witteberge and the Stormberg areas are included in the Drakensberg Grassland vegetation unit. The populations from the Witteberge are located in the Southern Drakensberg Highland Grassland (Gd4) and Lesotho Highland Basalt Grassland (Gd8), whilst the Stormberg population occurs in the Stormberg Plateau Grassland (Gd3). The central populations from the Toorberg, Andriesberg and Bowker’s Kop are included in the Sub-Escarpment Grassland vegetation unit, and the Queenstown Thornveld (Gs16) and the Tarkastad Montane Shrubland (Gs17) sensu Mucina & Rutherford=2006 (). The populations from the Great Winterberg are located in the Dry Highveld Grassland vegetation unit, and the Karoo Escarpment Grassland (Gh1). Finally, the southernmost population in the Amathole Mountains occurs in the Drakensberg Grassland vegetation unit and the Amatole Mistbelt Grassland (Gd2). These regions show bimodal spring-autumn or summer rainfall, usually with very dry winters, with precipitation ranging from 500–1300 mm, and an approximate mean of 50 days with frost per year (cf. Mucina & Rutherford 2006, Clark et al. 2014).
Distribution: —Known from several localities restricted to the Eastern Cape province of South Africa, from the Witteberge in the Southern Drakensberg near Lady Grey and Motkop in the north to the Great Winterberg–Amathole Ranges in the south around Cathcart and Tarkastad areas, being apparently restricted between the meridians 26− 28º E ( Fig. 10 View FIGURE 10 ). Therefore, the distribution of the new species can be included in the Drakensberg Alpine Centre of Endemism as redefined by Mucina & Rutherford=2006 (). The close related Massonia jasminiflora shows an allopatric distribution ( Fig. 10 View FIGURE 10 ), not known to us to occur in the Eastern Cape province, although some of their populations are located near the border to the latter province. The main distribution of M. jasminiflora is located in central and southern Free State province, with some populations extending to the adjacent areas in the North West province and Northern Cape province. It is worth mentioning that one apparently disjunct population of M. jasminiflora occurs in Mpumalanga ( Fig. 10 View FIGURE 10 ), near the golf course in Middelburg (Nieuwoudt 273 PRE), although the quarter degree given in the herbarium label (3125AC) seems to be mistaken with Middelburg from the Eastern Cape province. On this respect, Retief & Herman (1997) already cited M. jasminiflora (van Wyk coll. nº 928) from an area between Middelburg and the Waterberg in northeastern South Africa.
Morphological variation: —A certain variation on leaf morphology in Massonia amoena (under the name M. jasminiflora ) has been reported by Craib & Knoll (2000), especially regarding pustule size, colour and morphology. Craib & Knoll (2000) described the population at the type locality (Andriesberg) having always pustulate leaves, however some leaves were described as tinged with mauve or reddish brown and the pustules being ruby red, brown or mauvish brown. On the contrary, Craib & Knoll (2000) described the population from the Toorberg near Tarkastad as having nearly all plants pustulate leaves, but a few were plain with the surface merely tinged with various shades of mauve and sienna brown. Among the pustulate-leaved plants, specimens existed with big, evenly spread, mauvish pustules, some of them with congested ruby red pustules as well as other plants so densely pustulate that the leaf surfaces resembled coarse gravel sandpaper. It is also worth mentioning that the collection Galpin 1817 (K000257151!) from Bowker’s Kop, Queenstown, which is mounted together with the type of M. jasminiflora , includes four flowering plants that agree in flower morphology with M. amoena , but some of the pressed leaves apparently do not show evident pustules. However, a closer investigation of this collection reveals that the specimen situated at the bottom was mounted with the leaves showing the abaxial side and at least the plant placed on the left side show several small pustules on the leaves. Furthermore, a duplicate of Galpin 1817 (PRE0050981-0) includes 12 plants with strongly reflexed perianth segments and leaves showing evident remnants of pustules. In this regard, pressed leaves of Massonia amoena sometimes do not retain the morphology of the raised pustules, but remnants of them remain and the short papillae on top of each are visible. Craib & Knoll (2000) proposed that “the pustulate-leaved form, which is particularly cryptic, has probably evolved to mimic gritty dolerite soil. Evidence of this was found in the Toorberg plants, where very few had plain leaves and these were also either tinged with mauve or brown, causing them to blend better with the colours of the microhabitat”.
As a summary, all living specimens of M. amoena examined in this study showed evident pustules, being constantly heterogeneous in morphology from small and circular to elongated longitudinally, where a slight variation on pustule and leaf colour was detected ( Fig. 3 View FIGURE 3 ). However, in general terms, the leaf morphology of M. amoena can be considered as constant, especially regarding the diagnostic character differing from M. jasminiflora ( Fig. 1 View FIGURE 1 , 3 View FIGURE 3 ). We were not able to study living specimens of Massonia amoena with smooth leaves, as cited by Craib & Knoll (2000), but we suggest that these forms could be the outcome of possible hybridization processes with other species of Massonia bearing smooth leaves, such as for instance Massonia huttoni Baker (1870: 390) , M. versicolor Baker (1876: 184) and M. modesta Fourcade (1932: 79) , which also occur in the Eastern Cape Province of South Africa, and which are sympatric at least with some of the southernmost populations of M. amoena . However, a detailed study on this aggregate of taxa is necessary to evaluate this.
Additional specimens studied (paratypes): — SOUTH AFRICA. Eastern Cape. Lady Grey (3027 CB): Lady Grey, Joubert’s Pass , Alt. 2237 m, 20 February 2011, WW04486 ( GZU!); Lady Grey (3027 CD): Motkop, Alt. 2044 m, 20 February 2011, WW04485 ( GZU!); Lady Grey (3027): Majuba Nek , Herschel Distr. , January 1916, Hepburn 271 ( GRA!); Queenstown (3126 BA): Stormberg area (between Molteno and Dordrecht), Farm Streep Fontein 237, Alt. 1961 m, open rocky area on summit of ridge, growing among basalt boulders, rare, E. L . Gaisford & V. R . Clark 333 ( GRA!); Queenstown (3126 CD): in the foothills of the Toorberg , near Tarkastad, C . Craib & C . Knoll (Photo!); Queenstown (3126 DA): Andriesberg, Alt. 1777 m, 19 February 2011, WW04484 ( GZU!); Queenstown (3126 DA): Northern slopes Andriesberg, Alt. 5500 feet, May 1899, Fl. white, E. E . Galpin 2612 ( GRA!); Queenstown (3126 DB): Sterkstroom, Stapelbergskloof , Halseton, SW solpes of doleritic range, in cracks of rock, Alt. 6400 feet, 28 April 1963, C. R . Callaghan 32 ( PRE0050982 View Materials -0!); Queenstown (3126 DD): Bowker’s Kop, Queenstown , Alt. 4000 feet, May 1894, Fl. white, E. E . Galpin 1817 ( K000257151 !, PRE0050981 View Materials !); Lady Frere (3127 AC): Tafelberg, Dordrecht Distr. , on farm Blacks Siding, 25 May 1964, R . D. Bayliss 2237 ( PRE0050983 View Materials -0!); Lady Frere (3127 BC): Top of Cala Pass , 09 March 1987, Glen 1720 ( PRE0727264 View Materials -0!); Fort Beaufort (3226 AB): Great Winterberg ( Tarkastad District ), Farm Newtondale 228, cliffs and plateau edge, aspect NE , loamy clay, stony soil/rocky, grassland, dolerite, on cliffs, Alt. 1733 m, 18 March 2011, V. R . Clark & G . Neef 79 ( GRA!); Fort Beaufort (3226 AC): Along De Beers Pass , Great Winterberg ( Tarkastad District ), aspect S , hill slope, loam, stony soil/rocky, sandstone, rare, along the road cutting on pass, Alt. 1605 m, 17 March 2011, V. R . Clark & G . Neef 10 ( GRA!); Fort Beaufort (3226 AD): Great Winterberg Mountain , flat shelf on SW-facing slope, Alt. 1885 m, 24 May 2014, T . van Niekerk s.n. ( GRA!); Fort Beaufort (3226 BD); Amathole Mountains , ex. Simply Indigenous, WW 02266 ( GZU!) .
| E |
Royal Botanic Garden Edinburgh |
| GRA |
Albany Museum |
| CB |
The CB Rhizobium Collection |
| GZU |
Karl-Franzens-Universität Graz |
| BA |
Museo Argentino de Ciencias Naturales Bernardino Rivadavia |
| L |
Nationaal Herbarium Nederland, Leiden University branch |
| V |
Royal British Columbia Museum - Herbarium |
| R |
Departamento de Geologia, Universidad de Chile |
| C |
University of Copenhagen |
| DD |
Forest Research Institute, Indian Council of Forestry Research and Education |
| AC |
Amherst College, Beneski Museum of Natural History |
| BC |
Institut Botànic de Barcelona |
| NE |
University of New England |
| G |
Conservatoire et Jardin botaniques de la Ville de Genève |
| S |
Department of Botany, Swedish Museum of Natural History |
| AD |
State Herbarium of South Australia |
| T |
Tavera, Department of Geology and Geophysics |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |