Formica rufa emeryi STITZ, 1939
|
publication ID |
https://doi.org/10.25849/myrmecol.news_031:133 |
|
DOI |
https://doi.org/10.5281/zenodo.5587859 |
|
persistent identifier |
https://treatment.plazi.org/id/F52B87F6-5E25-6157-FC9F-DD6EFF751B59 |
|
treatment provided by |
Donat |
|
scientific name |
Formica rufa emeryi STITZ, 1939 |
| status |
|
Formica rufa emeryi STITZ, 1939 View in CoL
Formica rufa ab. emeryi STITZ, 1939 View in CoL [description and zoogeography]
STITZ (1939) made the first available use of F. rufa subsp. rufa ab. emeryi KRAUSSE, 1926 View in CoL . This taxon was described from near Eberswalde / Germany as specimens found within the nests of F.rufa View in CoL showing a colouration as in Formica pratensis View in CoL but with missing setae on eyes and hind tibia. Considering the species spectrum occurring in the vicinity of Eberswalde, these statements make a synonymy with F. rufa View in CoL most likely. Types are unknown.
All material examined. Numeric phenotypical data were recorded in 61 nest samples with 331 workers and 29 gynes; for details, see SI1, SI2, and SI3. The total number of mounted samples stored in SMN Görlitz and investigated either subjectively or by partial or complete numeric recording of the phenotypical characters used here was 103. These included 547 workers and 98 gynes and originated from Austria (two samples), Belgium (four), Bulgaria (six), Croatia (two), Finland (five), France (two), Germany (61), Greece (three), Italy (one), Norway (one), Poland (one), Russia (one), Slovenia (one), Spain (five), Sweden (four), and Switzerland (four). Character recording in ethanol-stored material according to the former investigation protocol of SEIFERT (1991) had been done until the year 1993 in further 196 nest samples with about 1600 workers, largely from Germany and Russia.
Geographic range. From Iberia east to Baikal region; probably a little more widely distributed than Formica polyctena due to the larger potency for long-range single-queen flight dispersal and socially parasitic colony foundation ( SEIFERT 1991, 2018). In Europe between 40.5° N ( Spain), 63.5° N ( Sweden), and 64.8° N ( Finland). A morphologically aberrant population exists in Asia Minor and Caucasus (here rare). The altitudinal distribution in European mountains is not well known. According to credible reports, it ascends in the Southern Alps ( 46° N) to 1500 m and in Asia Minor ( 40° N) to 1900 m.
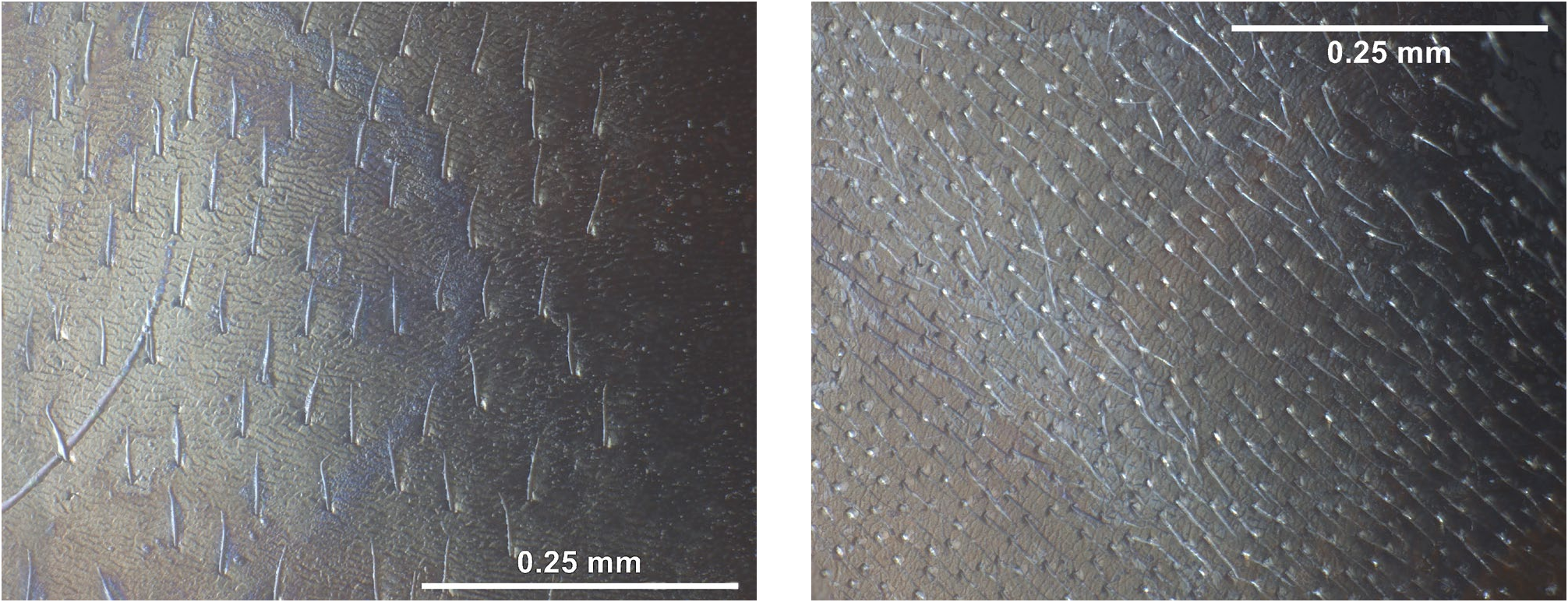
Diagnosis of worker ( Tab. 1 View Tab , key). Large; mean and maximum CS over all social types 1891 and 2274µm: Scape rather long and slender, SL / CS 1750 0.939, SL / Smax 10.10. Setae on eyes short, EyeHL 1750 22µm. Setae on posterior margin of head usually missing and, if present, rather short, nCH 1750 0.81, OccHL 1750 23 µm (a population in the montane region of Blanský Les, S Bohemia – labelled “ nespori ” in the collection of SMN Görlitz – has above-average values of nCH and OccHL but does not form a cluster sufficiently separate from Formica rufa when all characters are considered). Gular, pronotal, mesopleural and propodeal setae always present and rather long, nGu 1750 6.3, GuHL 1750 188 µm, nPn 1750 20.2, mPnHL 1750 81 µm, nMes 1750 15.4, nPr 1750 12.9; setae on metapleuron few or absent and of medium length, nMet 1750 1.9, MetHL 1750 144 µm. Pigmentation: Head with genae and its ventral surfaces always light reddish, dorsal head caudad from about transverse level of eye centers, the area between frontal carinae and surface along the frontal carina usually blackish brown; mesosoma light reddish, often with a medium-sized dark brown patch on dorsal pronotum. Specimens with nearly all surfaces of head, the mesosoma, and frontal face of first gaster tergite reddish, reminiscent of the condition in Formica truncorum , may occur, most frequently in large workers of the most hairy phenotypes.
Diagnosis of gyne ( Tab. 6 View Tab , Fig. 1 View Fig ). Medium-sized, CS 2140 µm; scape rather long and slender, SL / CS 0.868, SL / Smax 9.13. Setae on eyes short, EyeHL 25µm; setae on posterior margin of head always missing; gula without or with single setae of up to 280 µm length; pronotum without or single short setae of up to 44 µm length; meso- and metapleuron and frontal face of first gaster tergite without setae, if single setae are present these may have 200 - 244µm length; ventral surface of first gaster sternite usually with numerous long setae, nSt 18.8, StHL 350µm. Pubescence distance and distance of foveolae on paramedian dorsum of first gaster tergite rather high, sqPDG 9.00, FodG 57µm. Large parts of median and paramedian scutellum perfectly smooth and brilliantly shiny. Dorsum of gaster viewed at lower magnification always shiny. Pigmentation of head similar to worker; mesonotum, scutellum, and metanotum blackish brown; gaster black.
Taxonomic comments and clustering results. The nomenclatoric separation of Formica rufa and Formica polyctena is maintained here for pragmatic reasons but this decision is problematic according to the data presented in the next paragraph. This pragmatism follows a functional argument: In their pure expression, F. rufa and F. polyctena represent most different mor- phologies, types of ecological adaptation, dispersal, and reproduction strategies, which call for a different naming. Several hundred papers have been published so far using the name F. polyctena . Giving up the name F. polyctena would mean a loss of information and would complicate communication about biological issues.
A broad study considering 432 nest samples with 6100 worker ants and eight NUMOBAT characters and integrating intranidal phenotype composition as well as
topographic, ecological, and biological information ( SEIFERT 1991) provided evidence for frequent hybridization of Formica rufa and Formica polyctena . The adaptive advantage of the hybrid for conditions of fragmented forest systems hypothesized by the same author was made credible through mathematic modelling by HÖFENER & al. (1996). The presence of the supposed hybrid cluster in East Germany was later convincingly confirmed by a study integrating NUMOBAT characters and nuclear DNA data ( SEIFERT & al. 2010), with a high agreement of classifications provided by morphometrics and nuDNA (Fig. 21). Data on microsatellite DNA of GYLLENSTRAND & al. (2004) and SEIFERT & al. (2010) suggest that backcrossing of the hybrid and introgression occur mainly with the F. polyctena parent. This biased gene flow towards F. polyctena was also to be expected on the basis of differential queen acceptance and mating behaviour of the species (GÖSS- WALD 1942, SEIFERT 1991).
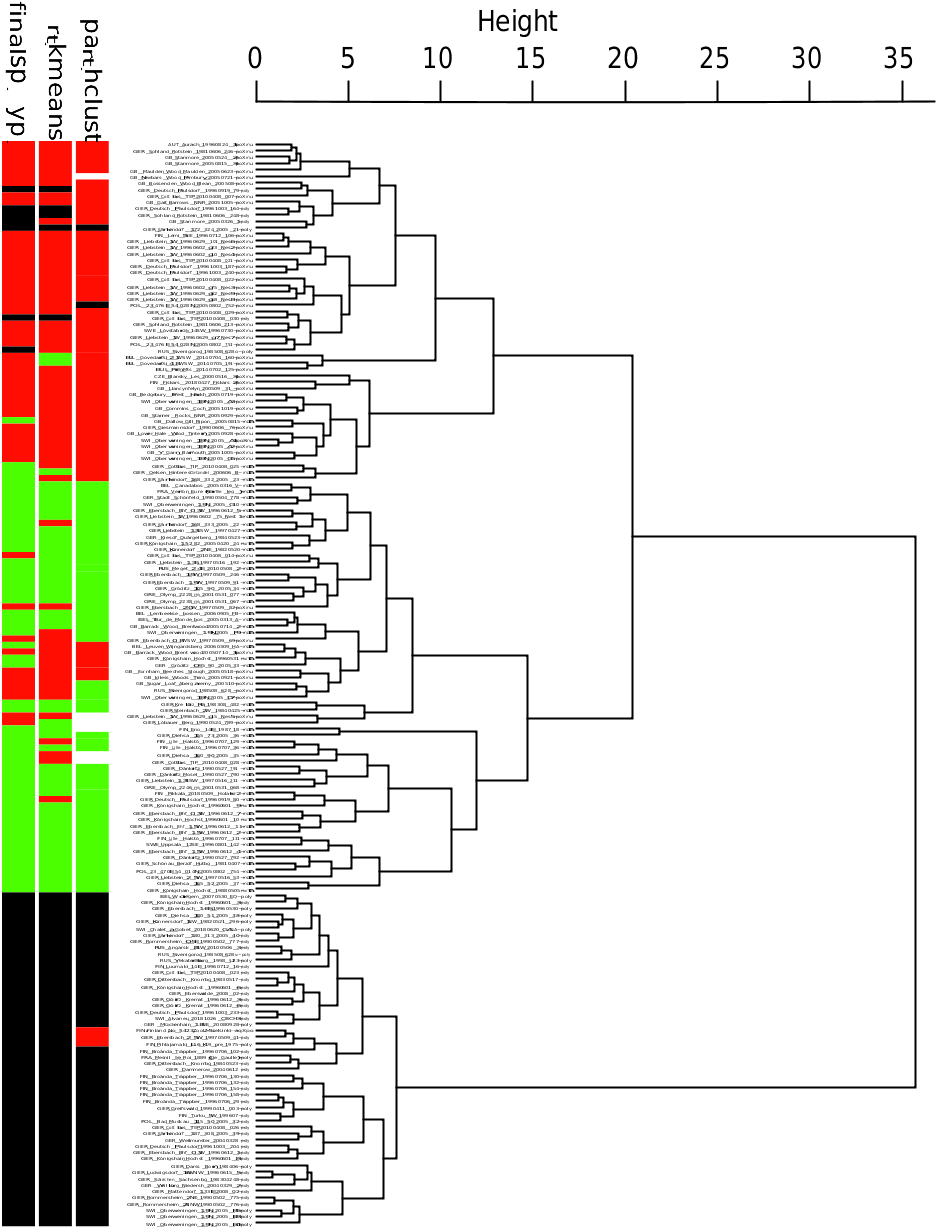
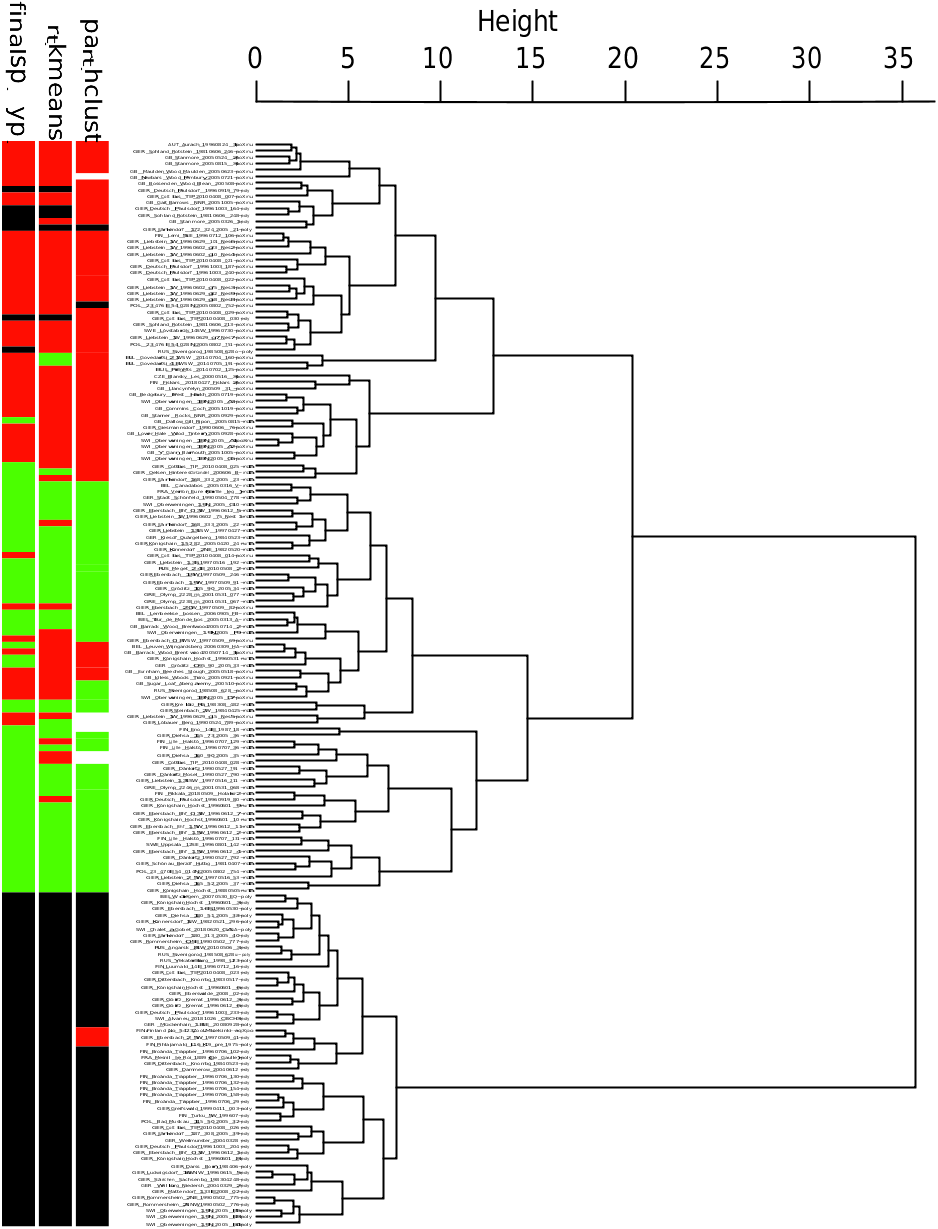
Hybrid frequencies of up to 28% for particular regions sum up to 6 - 8% over the whole European range and cause a dilemma in taxonomic decision making. A blind NC-clustering study of morphological data, ignoring any other source of information and the possibility of hybrid occurrence, provided misleading results ( Fig. 22 View Fig ). The figure considers 169 nest samples with 960 workers from entire Europe and the characters CS, CL / CW 1750, SL / CS 1750, nCH 1750, OccHL 1750, nGu 1750, GuHL 1750, nPn 1750, mPnHL 1750, nMes 1750, nMet 1750, MetHL 1750, and nPr 1750. Assuming K = 2 (two species and no hybrids present), the disagreement of the exploratory data analyses with the controlling LDA was 4.1% in NC-Ward, 5.9% in NCpart.hclust, and 1.2% in NC-part.kmeans. This means an average error rate of 3.7% and suggests acceptance of heterospecificity of Formica rufa and Formica polyctena following the <4% threshold recommended for NC-clustering by SEIFERT (2020a).Explicitly, NC-clustering confirms the F. polyctena cluster and a collective cluster formed by F. rufa and the hybrids. This clustering is largely explained by the high similarity of F.rufa and the hybrids in nGu 1750 and GuHL 1750 and a strong dissimilarity of F. polyctena regarding these characters. Assuming K = 3 (two parental species and a hybrid cluster), the disagreement is 13.6% in NC-Ward, 15.4% in NC-part.hclust, and 10.6% in NC-part. kmeans. Out of 53 hybrid samples and as mean of the three methods, 16.4% were allocated to the F. rufa and 2.5% to the F. polyctena cluster. These data show that the different algorithms of NC clustering cannot clearly expose hybrid samples and are likely to suggest a stronger divergence of the parental clusters than really given. A clear demonstration of hybrids with gaps to the parental clusters may be achieved in a vectorial space, either by running a PCA of RAV-corrected data or checking suspicious samples as wild-cards in an LDA. Yet, this proves only true when no or very few backcrosses are in the material ( SEIFERT 1984, 1999, 2006, KULMUNI & al. 2010, BAGHERIAN YAZDI & al. 2012, SEIFERT 2019a, b). In the present case, the LDA of the 169 samples ( Fig. 23 View Fig ) does not show obvious gaps separating the hybrid from the parental clusters, which may be explained by numerous backcrosses. The reader should be aware that the frequency of hybrids in this data set is about fivefold larger than the average figure expected for random sampling all over the European range. For hybridization with Formica lugubris , see section “Hybrids Formica lugubris × rufa ” (p. 174).
Biology. See the condensed information in SEIFERT (2018).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Formica rufa emeryi STITZ, 1939
| Seifert, Bernhard 2021 |
Formica pratensis
| RETZIUS 1783 |
Formica rufa ab. emeryi STITZ, 1939
| LINNAEUS 1761 |
F.rufa
| LINNAEUS 1761 |
F. rufa
| LINNAEUS 1761 |