Dasyurus geoffroui, Gould, 1841
|
publication ID |
https://doi.org/10.5281/zenodo.6608102 |
|
DOI |
https://doi.org/10.5281/zenodo.6602775 |
|
persistent identifier |
https://treatment.plazi.org/id/EA7087C1-FFB9-2455-FFC3-F3F50E1E0A87 |
|
treatment provided by |
Felipe |
|
scientific name |
Dasyurus geoffroui |
| status |
|
Western Quoll
French: Quoll de Geoffroy / German: Geoffroys Beutelmarder / Spanish: Dasiuro occidental
Other common names: Chuditch, Western Native Cat
Taxonomy. Dasyurus geoffroi Gould 1841 ,
Liverpool Plains , New South Wales, Australia .
In 1841, J. Gould described D. geoffroii from a specimen collected in New South Wales. In 1906, O. Thomas nominated two forms based on skin color, pelage, and skull size. In recent mitochondrial and nuclear genetic phylogenies, the quoll group (D. geoffrou, D. hallucatus , D. maculatus , and D. viverrinus ) form a monophyletic clade; the sister taxon to this group is the Tasmanian Devil ( Sarcophilus harrisii ). D. geoffroii has been placed as sister to D. spartacus from New Guinea and included in a moderately well-supported clade with D. spartacus and D. albopunctatus (also from New Guinea), to the exclusion of all other quolls. Genetic differentiation between D. geoffroii and D. spartacus is limited, less than 3% at the hypervariable mitochondrial D-loop, which is interesting given that even including historical distributions, apparently D. geoffroiz only occurred south of Cape York Peninsula (Queensland). Biogeographically, D. hallucatus is the only quoll that (albeit historically) occurred to the tip of Cape York; yet paradoxically, D. hallucatus and D. spartacus are genetically highly divergent. Because the nominate form, geoffroii , is apparently extinct, it is hard to test its validity, either genetically or morphologically. Two subspecies recognized.
Subspecies and Distribution.
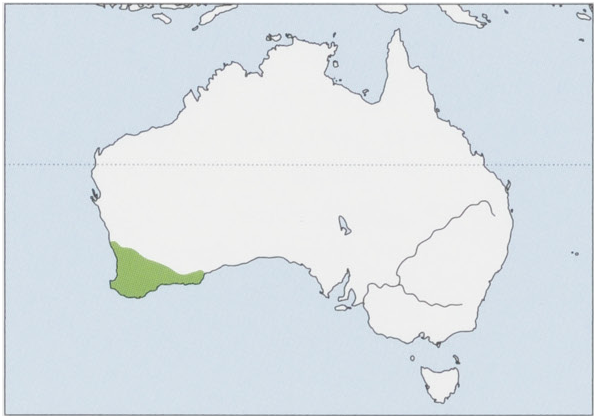
D. g. fortis Thomas, 1906 — SW Western Australia.
Successfully introduced (fortis) into Julimar Conservation Park, Western Australia. View Figure
Descriptive notes. Head-body 31-40 cm (males) and 26-36 cm (females), tail 25— 35 cm (males) and 21-31 cm (females); weight 0-71.2-2 kg (males) and 0-61.1-1 kg (females). There is marked sexual dimorphism for size. The Western Quollis distinguished from all other Australian and Tasmanian quolls by a bushy tail that is black on the distal half. Body color is brownish-gray above, with conspicuous white spots; underside is creamy-white.
Habitat. Wide range offorest, shrub, and desert habitats across much of mainland Australia, apart from the south-eastern coast and the Top End. The subspecies fortis occurs in areas dominated by sclerophyll forest, drier woodland, heath, and mallee shrubland in south-western Western Australia.
Food and Feeding. The diet of Western Quolls in deserts (subspecies geoffroit) include mammals up to at least the size of a European Rabbit (Oryctolagus cuniculus), lizards, frogs, and invertebrates. In forest habitats, Western Quolls consume insects (winged termites to large cockroaches and beetles), freshwater crustaceans, and a variety of terrestrial reptiles, mammals, and birds (up to the size of bandicoots and parrots). Western Quolls sometimes climb small trees to obtain prey, clasping trunks between their long hindfeet and gripping with forepaws. One study in the Jarrah forest of Western Australia found that mammals and invertebrates dominated the diet. Reptiles and birds were also consumed frequently, confirming the Western Quoll as a generalist predator. A high proportion of large mammals found in diets of Western Quolls, such as Common Brush-tailed Possums (Trichosurus vulpecula) and Southern Brown Bandicoots (Isoodon obesulus), suggests that it may also be a frequent scavenger. Diets of Western Quolls varied seasonally, with reptiles and invertebrates eaten more frequently during warmer months.
Breeding Litters of Western Quolls are born in May-September, most appearing in June-July. Up to six newborn young are accommodated in the enclosed pouch. Juveniles protrude through the pouch opening at 6-7 weeks old; young at c.9 weeks of age have outgrown the pouch entirely and are left in a den while their mother forages. At this age, young are vulnerable; they can barely crawl, are poorly insulated by fur, and are unable to shiver. By c.16 weeks of age, juvenile Western Quolls are well furred and begin to eat solid food. Young are weaned by 22-24 weeks and typically disperse shortly after this time in summer. Males and females are capable of breeding at c.1 year of age. About 80% of weaned juveniles are evidently offspring of first-year mothers. In part, this reflects the fact that first-year females comprise slightly more than one-half of breeding females in a population. First-year females tend to have largerlitters than older females; indeed, they are about three times more successful in raising young to the age of weaning. One study examined growth and development of young in captive and wild litters of Western Quolls. Litters were monitored at 1-5 day intervals from birth until they were left in dens at 62-72 days of age. Two neonates were 0-44 cm long and weighed an average 0-01 g; by 63 days of age, juvenile weight had increased an astonishing 1500fold to reach an average of 16:7 g. Growth appeared to be roughly linear with time. Wild Western Quolls were first left in dens at the age of 62 days, soon after outgrowing confines of the pouch. Wild and captive growth rates appeared similar through the first one-half of pouch life. Nevertheless, at older ages, wild litters generally grew more slowly than captive litters; indeed, wild litters belonging to thin mothers grew more slowly than litters with medium-weight mothers, which in turn grew more slowly than litters of fat mothers.
Activity patterns. Western Quolls forage primarily on the ground and at night, but the species is sometimes active during the day, especially at the height of the breeding season or when cold, wet weather serves to restrict nocturnal foraging.
Movements, Home range and Social organization. The Western Quoll occurs at low densities even in high-quality habitat. For example, along the Murray River valley in Western Australia, adult females denned within a stable core area of 55-120 ha; male dens were distributed over more than 400 ha. Core areas of neighboring females typically showed little or no overlap, suggesting they are actively defended. In contrast, male core areas overlapped extensively with those of males and females. In one study of spatial requirements in south-western Western Australia, radio tracking resulted in 123 locational fixes that included 69 different refuge sites. Number of locations recorded for each Western Quoll ranged from nine to 38. Mean maximum distance moved by individuals between refuges was 1-5 km for females and 7-9 km (range 4-5—-12 km) for males. Average distance traveled by Western Quolls between consecutive refuge sites was 0-5 km for females and 3-3 km for males. Radio-tracked females tended to maintain exclusive core home ranges. Core home ranges of males exhibited a large degree of overlap, with other males and females. Males were also recorded using refuges used by other males and females, with five refuge sites recorded as being used by more than a single individual. Two males used one such refuge, a rocky outcrop, at the same time, but they were mostlikely using separate crevices. In another study, juveniles first began exploring outside their natal den at c.17 weeks of age and thereafter rapidly increased their foraging duration and distance. By the time weaning was completed during weeks 22-24, juveniles devoted at least six hours to an initial foraging bout and traveled more than 500 m from their dens nightly. Initial phase of dispersal occurred soon after weaning; juveniles denned separately from their mothers but remained in their maternal home range. Mothers sometimes instigated this phase by simply abandoning their litters in the natal den. Second phase of dispersal from the natal area was strongly male-biased, occurring when juveniles were 25 weeks old on average and covered long distances (more than 10 km). Most females were philopatric or settled in vacant areas near their maternal home range. Observation of captive Western Quolls during weaning indicated that social play among littermates might be important at this time. Aggressive wrestling rapidly escalated during initial weaning when juveniles were active but in close proximity for most of the night. Intensity of wrestling declined before weaning was complete. Wild juveniles also apparently participate in wrestling bouts, although possibly on a more limited scale than captive individuals, which suffer from enforced proximity. This play behavior may facilitate social cohesion in the litter at a time when juveniles are first capable of causing each other harm, or at least it provides practice for fighting techniques used later by adults to defend territories and secure mates.
Status and Conservation. Classified as Near Threatened on The IUCNRed List. The subspecies fortis is listed as vulnerable in Australia, and D. g. geoffroii is presumed extinct. Western Quolls have suffered major distributional retraction since European settlement of Australia (1788). The Western Quoll was once distributed over nearly 70% of the Australian mainland, but its distribution has now contracted to less than 2% of the continent. Museum specimens were last collected in New South Wales in 1841, Victoria in 1857, Northern Territory in the early 1900s, and Queensland no later than 1907. The most recent substantiated reports of the Western Quoll in the central deserts of Australia date from the mid-1950s, but there arestill a few unconfirmed reports from the 1980s and 1990s in western New South Wales. The subspecies fortis was determined to be declining in the wheat belt, but translocations had at least in part reversed this trend despite a continued decline in several populations. Stability of populations currently depends on active management. Threats to the Western Quoll remain, butlikely at lower levels than in the past It now occurs at low densities throughout the Jarrah forest and more patchily in the drier woodlands and mallee shrublands of the central and southern wheat belt. Although Western Quolls have been known to live for more than five years in captivity, wild individuals rarely survive more than c.3 years. Monitoring of radio-tagged individuals indicates that many factors likely contribute to mortality, including collision with motor vehicles, illegal shooting, predation by raptors and Red Fox (Vulpes vulpes), disease and natural incidents such as drowning, and injuries suffered in leg-hold traps set for Red Fox or European Rabbits around poultry sheds. Results of live-trapping surveys indicated that fewer than 6000 Western Quolls survived in the wild in the late 1980s. A recovery plan was established in 1991. One recent study on relationships between the Western Quoll and introduced predators found thatintroduced predators are indeed a source of mortality, but the extent of this mortality and relative contribution of each predator species remain unclear. Capture rates of Western Quolls also increased following control of red fox, but a conclusive link with abundance of Western Quolls has yet to be demonstrated, and the mechanism behind this response is also obscure—so there is much left to learn to enable sustainable management. Population of Western Quolls certainly exist in areas with no control of red fox, but it is unknown if these represent sinks into which individuals disperse from nearby baited areas. It is noteworthy that diet and habitat use of Western Quolls are similar to those of several other native and introduced predators. Nevertheless, overlap in resource use has not been studied in sympatric populations and resource use has not been compared among areas with or without control of red fox.
Bibliography. Firestone (2000), Glen, de Tores et al. (2009), Glen, Wayne et al. (2010), Gould (1841b), Mor ris et al. (2003), Rayner et al. (2012), Serena & Soderquist (1988, 1989a, 2008), Soderquist & Serena (2000), Thomas (1906).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
SubClass |
Metatheria |
|
Order |
|
|
Family |
|
|
Genus |
Dasyurus geoffroui
| Russell A. Mittermeier & Don E. Wilson 2015 |
Dasyurus geoffroi
| Gould 1841 |