Desmodium incanum, DC.
|
publication ID |
https://doi.org/ 10.1016/j.phytochem.2016.02.013 |
|
DOI |
https://doi.org/10.5281/zenodo.10530339 |
|
persistent identifier |
https://treatment.plazi.org/id/E9788781-A153-FFE1-E936-F900FD22F97E |
|
treatment provided by |
Felipe |
|
scientific name |
Desmodium incanum |
| status |
|
2.6. Synthesis of 6,8-di-C-glycosylflavones using 6-C-glucosyl-2- hydroxyflavanone substrates created by OsCGT and CGT enzymes from D. incanum View in CoL
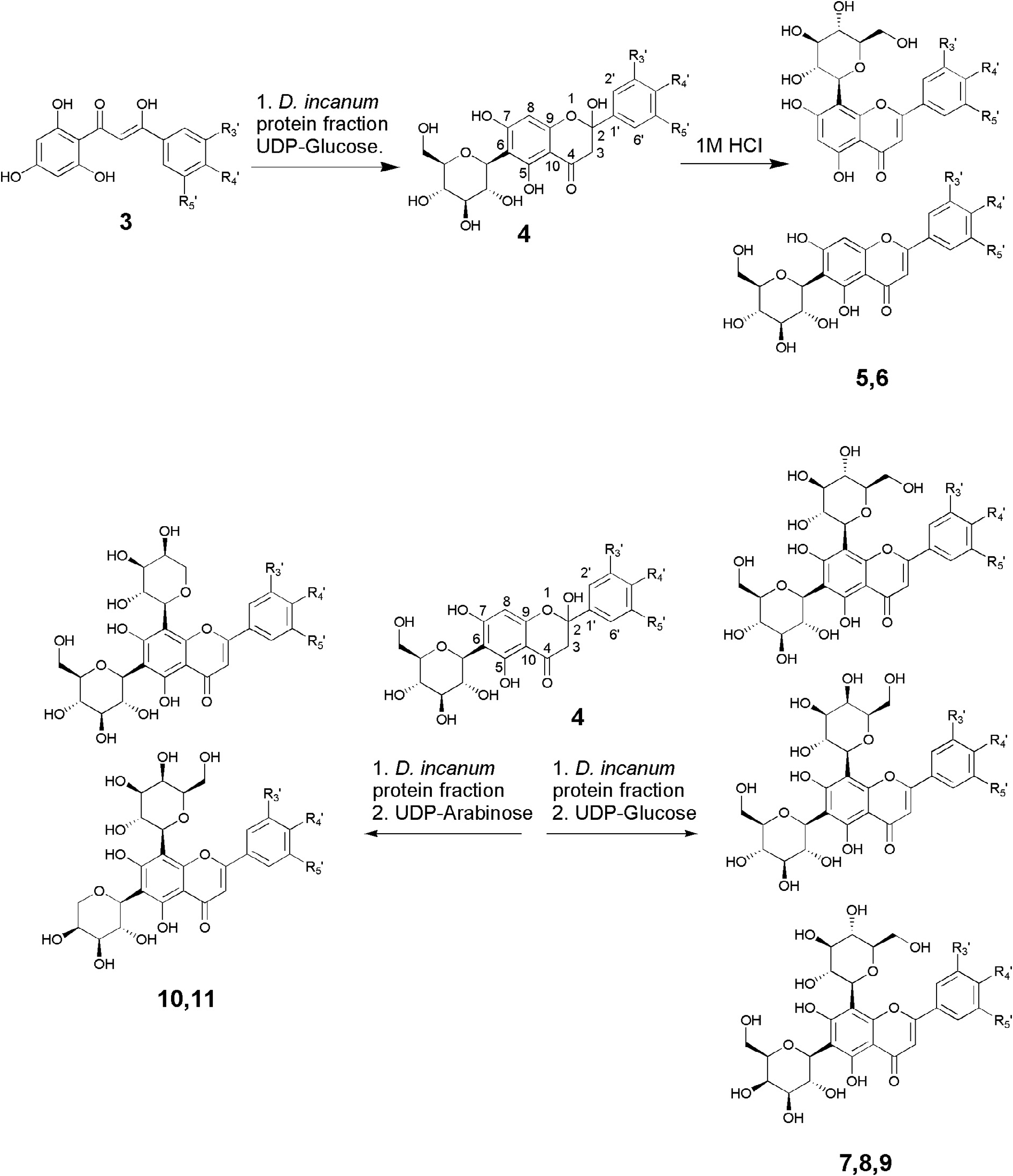
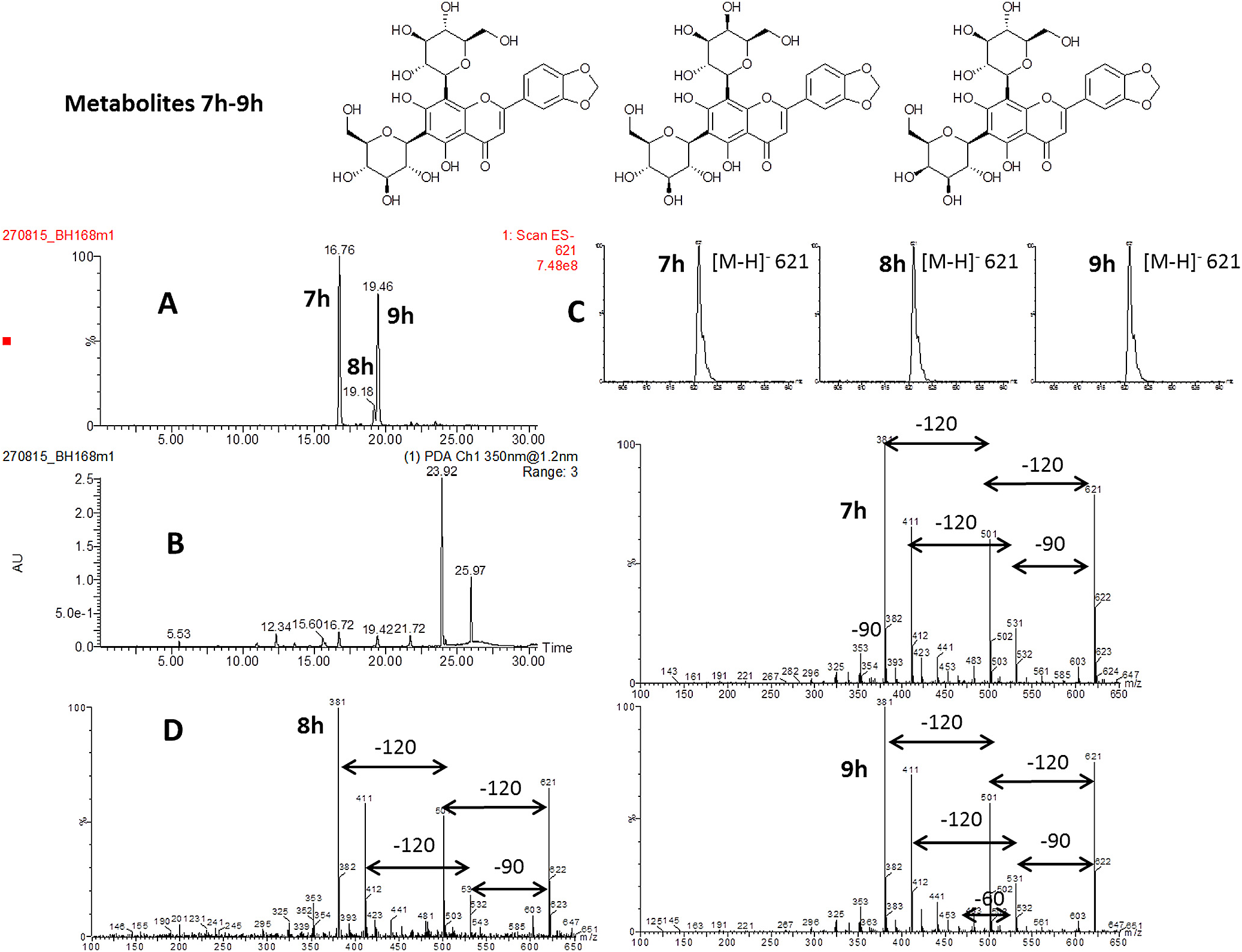
The results of incubating synthetic 2-hydroxyflavanones with UDP-glucose and OsCGT ( Tables 1 View Table 1 and 2 View Table 2 ) revealed substrate that can be used to generate C -glucosyl-2-hydroxyflavanones (4) that may be themselves substrates for a second glycosylations. In our work to understand the biosynthesis of di- C -glycosylflavones in the allelopathic root exudates of D. incanum we have demonstrated that the soluble plant enzyme extracts can C -glucosylate, C -galactosylate and C -arabinoslyate the mono- C -glucosylflavanones 4a to produce di- C -glycosylflavonones that dehydrate to the respective flavones ( Hamilton et al., 2012; Hao et al., 2015). We therefore used synthetic 2-hydroxyflavanones that are well accepted by the OsCGT to generate natural and novel C -glucosyl-2-hydroxyflavanones ( Table 3 View Table 3 ). By using recombinant OsCGT, these unstable intermediates can be generated in good yields and purity without the need for separation from other flavonoids. The incubation of the substrates with UDP-glucose is expected to give three products (7–9), rather than one containing two glucose groups, as the D. incanum soluble enzyme extract is able to isomerise UDP-glucose to UDP-galactose which is subsequently incorporated and after dehydration can be fixed in either the C- 6 or C- 8 position to give two regiosiomers ( Scheme 1 View Scheme 1 ) ( Hao et al., 2015). The incorporation of UDP-arabinose likewise produces two products (10, 11), regioisomers with the C -arabinose in either the 6 or 8 position. In all cases, where the correct number of novel products was detected with the correct molecular weight, the fragmentation pattern was subsequently carried out under high cone voltage ESIMS conditions to demonstrate that the products are indeed di- C -glycosylflavones. In the case of C -hexosylflavones, loss of 90 and 120 amus indicate the correct C -link and with C -arabinosylflavones, loss of 60 and 90 amus is indicative of the correct structure ( Fig. 1 View Fig ). The results of these experiment showed that there was a greater relaxed specificity for C -glucosylation and C -galactosylation than for C -arabinosylation. Substrates 4a, b, d, f, g, h, k,o,p,s all produced 3 new metabolites when incubated with UDP-glucose and D. incanum root protein, deduced to be structures 7, 8 and 9 by ESIMS mass spectrometry (Supplementary data). In the case of 4b, as the products are already present in D. incanum and are co-extracted with the proteins, the labelled sugar moiety UDP-α- D-[UL- 13 C 6]glucose was used and incorporation judged by presence of the labelled [ M — H +6] — 615 ion with six 13 C atoms. The ion trace, low cone voltage ESIMS spectrum and fragmented high cone voltage ESIMS spectra from compounds 7h, 8h, and 9h produced from 4h are shown ( Fig. 3 View Fig ). The selective ion trace at 621 amus ( Fig. 3A View Fig ) shows three new metabolites with the correct ion ([ M — H] — 621) which was shown to be the anticipated molecular ion ( Fig. 3C View Fig ). Fragmentation shows sequential losses of 90 ([ M — H — 90] — 531 and [ M — H — 180] — 441} and 120 amus ([ M — H — 120] — 501 and [ M — H — 240] — 381) and combinations of losses ([ M — H — 90-120] — 411 ( Fig. 3D View Fig ) indicating both sugars must be C -linked to the apigenin core ( Fig. 1 View Fig ). This has previously been shown for 4a where the three new metabolites produced were characterised through the NMR characterisation of natural standards as the di- C -glucosylated compound and two C -galactosylated regioisomers 7a, 8a and 9a ( Hooper et al., 2015). The substrates that were not incorporated were 4e and 4i which both possess two oxygen atoms in meta positions. The more sterically bulky substrate 4l was also not C -hexosylated. In the case of C -arabinosylation, however, there was a distinctly lower tolerance for different B-ring functionality with products only generated from substrates 4f, 4h and traces from 4g, all very close in structure to the natural substrate 4a. These three substrates were incorporated to yield 10f, g, h and 11f, g, h (Supplementary data). Representative data ( Fig. 4 View Fig ) reveal two compounds in the ion trace ( Fig. 4B View Fig ) with ions of 591 ( Fig. 4A View Fig ) which are revealed as molecular ions [ M — H] — 591 ( Fig. 4C View Fig ) and which fragment to give losses of 60 ([ M — H — 60] — 531, losses of 90 [ M — H — 90] — 501}, losses of 120 ([ M — H — 120] — 471 and combinations of losses ([ M — H — 60-90] — 441, [ M — H — 60-120] — 411 and [ M — H — 90-120] — 381 ( Fig. 4D View Fig ) indicating both sugars must be C -linked to the apigenin core ( Fig. 1 View Fig ).
| C |
University of Copenhagen |
| M |
Botanische Staatssammlung München |
| H |
University of Helsinki |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.