Caridina biyiga, Short & Page & Humphrey, 2019
|
publication ID |
https://doi.org/10.11646/zootaxa.4695.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:54231BE1-08B9-492E-8436-3A3B1EF92057 |
|
persistent identifier |
https://treatment.plazi.org/id/31FA6024-ADA6-4DB9-8267-22737F45B23D |
|
taxon LSID |
lsid:zoobank.org:act:31FA6024-ADA6-4DB9-8267-22737F45B23D |
|
treatment provided by |
Plazi |
|
scientific name |
Caridina biyiga |
| status |
sp. nov. |
Caridina biyiga View in CoL sp. nov.
( Figs 2–5 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 , 7–9 View FIGURE 7 View FIGURE 8 View FIGURE 9 ; Tables 1–3 View TABLE 1 View TABLE 2 View TABLE 3 )
Type Material. Holotype, MAGNT Cr 019223, ♀ ( 2.2 mm CL), Leichhardt Springs, north arm, Kakadu NP, North- ern Territory, Australia, 12°46.6’ S, 132°51.5’ E, L. Chandler, D. Buckle, 26/5/2009 GoogleMaps . Paratypes, MAGNT Cr019224, 5 ovig. ♀, ( 2.15–3.05 mm CL), same collection data as holotype GoogleMaps ; MAGNT Cr019225, 2 ♂ ( 2.05–2.15 mm CL), same locality as holotype GoogleMaps , SSB, 14/5/2003; MAGNT Cr019226, 2 ovig. ♀ ( 2.45–2.7 mm CL), same collection data GoogleMaps ; MAGNT Cr019227, 1 non-ovig. ♀ ( 2.25 mm CL), same collection data GoogleMaps .
Non-type material: MAGNT Cr 019228, 47 unsexed, Leichhardt Springs, south arm, Kakadu NP, Northern Territory, Australia, 12°46.7’ S, 132°51.3’ E, M. Hammer, M. Ellis, 8/6/2016 GoogleMaps ; MAGNT Cr 019229, 2 unsexed, Leichhardt Springs, downstream confluence of north and south arms, Kakadu NP, Northern Territory, Australia, 12°46.5’ S, 132°51.3’ E, M. Hammer, M. Ellis, 8/6/2016 GoogleMaps .
Comparative material: Caridina wilkinsi , MAGNT Cr 019230, 1 ovig. ♀ ( 2.5 mm CL), Sandy Creek below Sandy Creek Falls , Northern Territory, 13°44.66’ S, 130°44.66’ E, NT MRH, Stn FN 01, Water Res. Div. LPE, 30/10/1996 GoogleMaps .
Caridina typus, MAGNT Cr 019231, 1 ♀ ( 3.2 mm CL), Ira Lafai, spring source, Timor-Leste , ERISS, 10/9/2006 .
Caridina ‘sp. Gulf1’, MAGNT Cr 019232, 1 ♀ ( 2.75 mm CL), Baralil Billabong, Kakadu NP, Northern Terri- tory, ERISS, 29/5/2006 .
Caridina ‘sp. NT1’, SAM 7904, 5 unsexed ( 1 specimen sequenced for Page et al. 2007a), Melville Island, Northern Territory, in creek approx. 2 km from river mouth, C. Watts, 23/5/1999; MAGNT Cr 019233, 1 ovig. ♀ ( 3.25 mm CL), Takamprimili Creek downstream of Pickertaramoor Airstrip , Melville Island, Northern Territory, 11°46.9’ S, 130°52.7’ E, NT MRH, stn ML03 , Water Res. Div. LPE, 17/9/1996 GoogleMaps .
Caridina ‘sp. NT2’, MAGNT Cr019234, 1 unsexed ( 2.25 mm CL), Casuarina Coastal Reserve, Tiwi Stormwater Drain, Darwin, Northern Territory, 12°21.9’ S, 130°52.4’ E, NT MRH, Stn DW12, Water Res. Div. LPE, 1/10/1996.
Caridina ‘sp. NT nilotica’, MAGNT Cr019235, 1 ♀ ( 7.25 mm CL), Leanyer Swamp, Buffalo Creek, Northern Territory, D. Wilson.
Caridina ‘sp. WA4’, MAGNT Cr019236, 1 unsexed ( 3.9 mm CL), Howard River at Gunn Point road crossing, Northern Territory, D. Wilson.
Etymology. An indigenous word from the Gundjeihmi language (pronounced bee-yee-ga) meaning ‘different’ or ‘other’, a reference to its unusual morphology compared to other species of Caridina . To be used as a noun in apposition.
Proposed vernacular name. Leichhardt Springs shrimp.
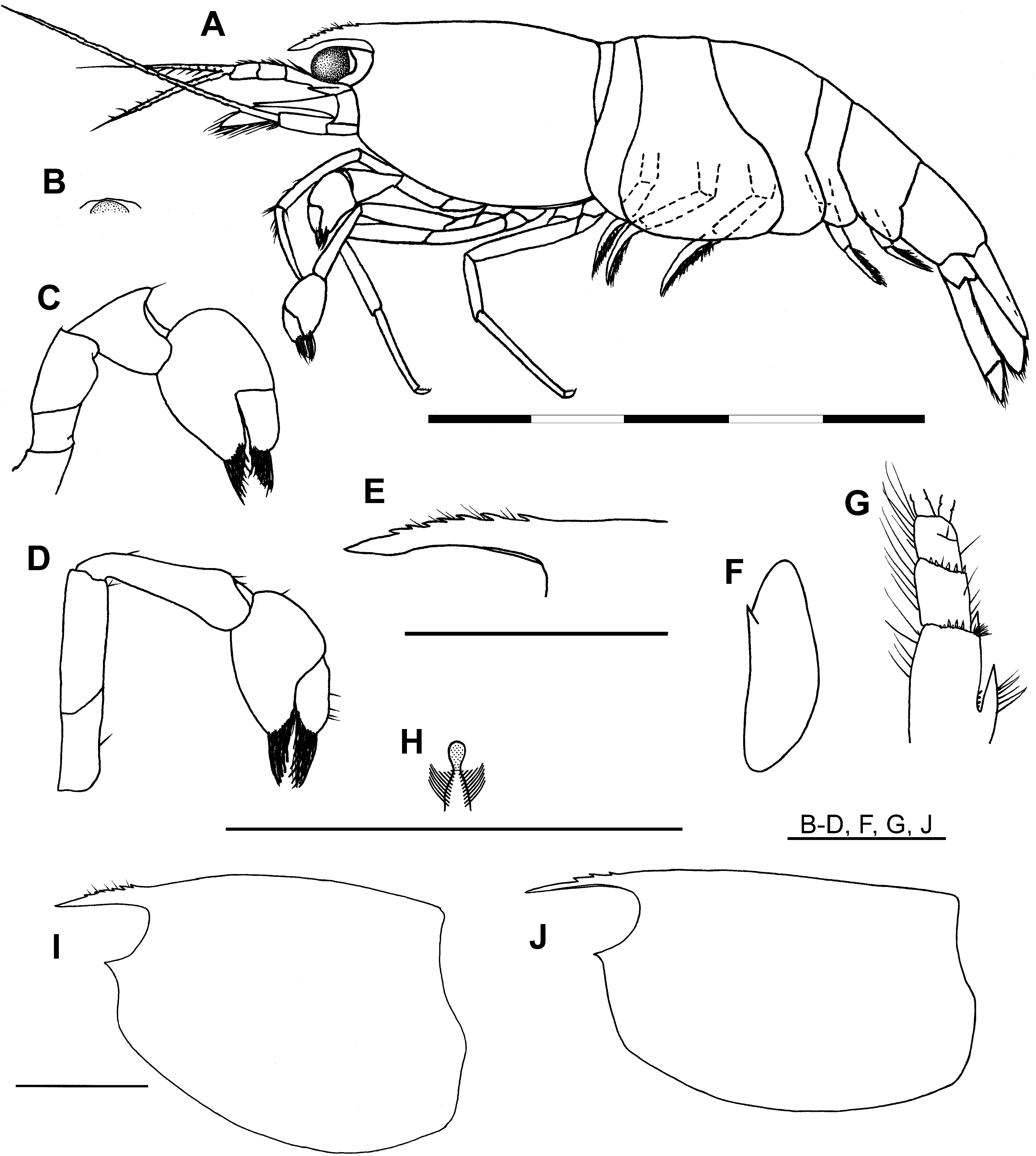
Description. Habitus ( Fig. 2A View FIGURE 2 ). Small, subcylindrical, rotund species of the genus. Maximum size of females 3.05 mm CL ( n = 9); males 2.15 mm CL ( n = 2); minimum size of ovig. females 2.15 mm CL ( n = 7).
Acron ( Figs. 2 View FIGURE 2 A–B). Median carina absent, anteromedially with low, rounded, transverse carina, anteromedian projection (bec ocellaire) absent. Eyes well developed, cornea large, hemispherical, well pigmented.
Carapace ( Figs. 2A, E View FIGURE 2 , I–J). Smooth, glabrous; dorsum slightly to moderately humped in adult females, indistinctly humped in males. Rostrum short, at most reaching proximal 1/3 of intermediate antennular peduncle segment, 0.3–0.4 × CL ( x = 0.35, n = 11); slender, depressed, appearing triangular in dorsal view; lateral carina well developed, ventral carina obsolete, dorsal carina generally poorly developed, less commonly moderately developed; apex acute; dorsal carina slightly convex to almost straight, armed with 3–7 preorbital teeth (commonly 4–6), teeth variably distributed on dorsal margin, commonly unarmed near tip, interspaces between teeth setose; ventral carina unarmed. Antennal spine well developed, completely fused with, and indistinct from, inferior orbit. Pterygostome broadly rounded.
Antennule ( Fig. 2G View FIGURE 2 ). Peduncle short, failing to reach distal margin of scaphocerite; distal margins of basal and intermediate segments with row of short spiniform setae; anterolateral angle of basal segment acutely pointed, reaching to around 1/3 length of intermediate peduncle segment; stylocerite short, failing to reach distal end of basal antennular peduncle segment, ca. 0.2 × CL.
Scaphocerite ( Fig. 2F View FIGURE 2 ). Short, 0.6–0.65 × CL, length 2.75–3.0 × breadth, tapering strongly from broadest point to anterior margin, broadest point in proximal half, strongly produced anteromedially, lateral margin slightly concave.
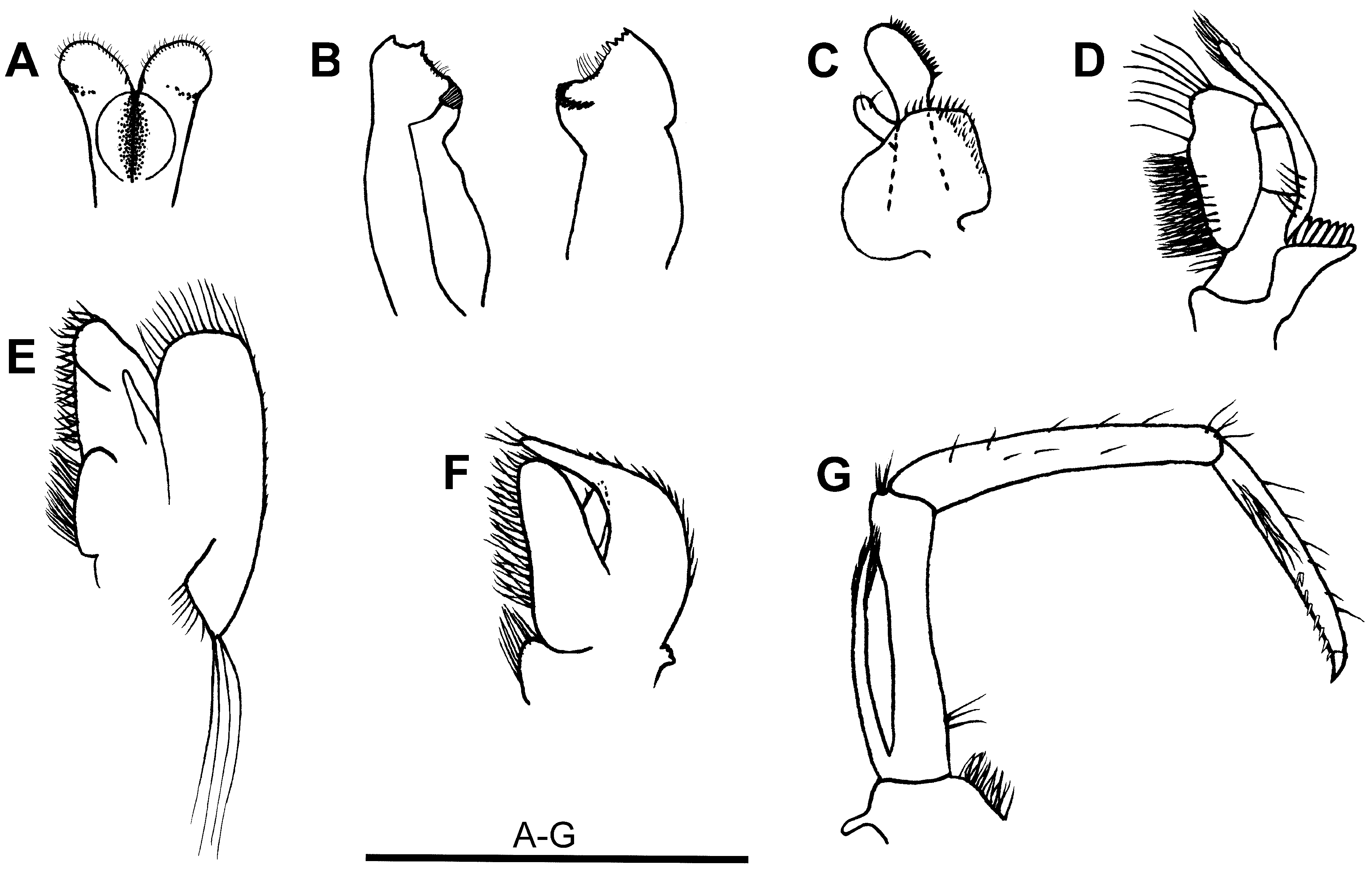
Mouthparts ( Figs. 3 View FIGURE 3 A–G). Labrum broadly rounded anteriorly, asetose. Metastoma with paragnatha incompletely fused basally to form weak corpus, basal paragnath with laterally revolute, semicircular carina, distolateral paragnath feebly alariform, distal margin rounded, distal and distomesial margins setose. Mandibles with well-developed incisor and molar processes, dimorphic, left and right mandible of similar size but differing in shape, left mandible with incisor process bearing 2 larger outer cusps and smaller, indistinct inner cusp, patch of long setae along margin leading to molar process, molar process strongly produced, ridged, right mandible incisor process with 2 outer cusps larger than inner pair, inner margin leading to molar process with 3 spiniform setae followed by patch of long setae, molar process ridged in semicircular arc. Maxillula with upper lacinia broadly elongate, mesial margin straight, with row of spiniform setae, lower lacinia bluntly angular distomesially, setose along distal margin and mesially; palp well developed, entire, bisetose, slightly expanded distally. Maxilla with distinct basal and coxal endites, inner margins densely setose; palp slender, tapering; scaphognathite well developed, with plumose setae distally, shorter setae laterally, proximal triangular process short and broad, terminating in several very long setae. First maxilliped with well separated basal and coxal endites, inner margins densely setose; palp broad, triangular, distally setose; caridean lobe well developed; rudimentary epipod present; exopod well developed, flagellum distinct, with few distal plumose setae. Second maxilliped with dactylar and propodal segments fused, slightly concave and densely setose mesially; coxa asetose, slightly produced mesially; exopod narrow, short, just over-reaching propodus, with marginal plumose setae distally; mesial margins of merus and ischium sparsely setose; carpus with few distal setae. Third maxilliped reaching slightly beyond scaphocerite; terminal segment ca. 0.8 × penultimate segment length, ending in sharply hooked unguis followed by row of ca. 9 large spiniform setae on distal third of segment; ischiomerus and basis fused, ischiomerus with few spiniform setae distolaterally; exopod a little shorter than ischiomerus, with long plumose setae on distal margin.
Branchiae ( Figs. 3D View FIGURE 3 , F–G; 4J; Table 3 View TABLE 3 ). Epipod rudimentary on first maxilliped, well developed on maxillipeds 2–3 and P1–4, bilobed on maxilliped 3 with bluntly elongate anterodorsal lobe and strap-like posteroventral lobe bearing distal hook, strap-like with distal hook on P1–4, bisetose setobranchs present on P1–5, well-developed, elongate, 6-lamellar podobranch present on epipod of maxilliped 2. Exopods present on maxillipeds, absent on pereiopods. Two arthrobranchs above maxilliped 3 (upper arthrobranch small, lower arthrobranch well developed), arthrobranch absent above P1. Pleurobranchs well developed above P1–5.
First cheliped ( Figs. 2C, H View FIGURE 2 ). Short, at most reaching distal margin of intermediate segment of antennular segment; chela short, length 1.2–1.4 × breadth, fingers with flexible, sclerotinous, spatulate tips, dactylus slightly shorter to equal in length to manus; carpus deeply excavated distally for reception of chela, length 0.9–1.3 × breadth, 0.65–0.9 × chela length, equal in length to or slightly shorter than merus; merus compressed.
Second cheliped ( Fig. 2D View FIGURE 2 ). Longer and slightly more slender than first pereiopods, reaching terminal segment of antennular peduncle; chela short, length 1.4–1.9 × breadth, dactylus about equal in length to clearly longer than manus, fingers with flexible, sclerotinous, spatulate tips; carpus slightly excavated distally for reception of chela, subconical, length 3.0–3.7 × breadth.
Third pereiopod ( Fig. 4E View FIGURE 4 ). Short, distal third of propodus reaching distal scaphocerite; dactylus short, broad, length 2.1–2.3 × breadth, flexor margin armed with 3–4 spiniform setae generally decreasing in size proximally, distal spiniform seta sometimes subequal to unguis, unguis moderately developed; propodal length 7.7–9.1 × max. breadth, 4.7–4.8 × dactylar length, with 2 rows of moderate-sized spiniform setae along flexor margin; carpus with well-developed distal projection; merus with 2 large spiniform setae on flexor margin; coxa with semicircular, concave, lateral plate.
Fourth pereiopod ( Figs. 4F, J View FIGURE 4 ). Short, reaching distal half of intermediate segment of antennular peduncle; dactylus short, broad, length 2.4–2.5 × breadth; propodus bearing 1 large spiniform seta on flexor margin, length 7.7–9.1 × max. breadth, 4.6–6.0 × dactylar length; coxa with semicircular, concave, lateral plate.
Fifth pereiopod ( Fig. 4K View FIGURE 4 ). Short, failing to reach distal margin of basal antennular segment; dactylus similar to third and fourth pereiopods, short, length 2.1–2.4 × breadth, flexor margin armed with 3–4 spiniform setae, unguis feebly to moderately developed; propodus with 9–11 moderately large, spiniform setae on flexor margin, length 9.6–10.8 × max. breadth, 5.8–6.1 × dactylar length.
Abdomen ( Figs. 4 View FIGURE 4 A–D, G). Smooth, glabrous, evenly rounded in lateral view; first to third pleura broadly rounded posteroventrally; fourth pleura produced and rounded posteroventrally; fifth pleura produced and bluntly angular posteroventrally, length ca. 0.5 × sixth pleura length; sixth pleura with posteroventral angle bluntly angular, posterolateral angle acute, non-spinate, length ca. 0.45 × CL, ca. 1.4 × depth. First male pleopod with endopod length ca. 0.5 × exopod length, ca. 2.0 × breadth, triangular, apex blunt, lateral margin setose, mesial margin with single seta; without appendix interna. Second male pleopod with endopod length 0.85–0.9 × exopod length; appendix interna reaching to about half appendix masculina length, with numerous retinaculae distally; appendix masculina spatulate, length 0.4–0.5 × endopod length, bearing ca. 11–13 long spiniform setae on mesial and distal margins. Uropodal exopod with well-developed diaeresis, diaeresis bearing 12–16 spiniform setae. Uropodal protopod with acutely pointed, short, posterior projection, protopod length 1.15–1.25 × breadth.
Telson ( Fig. 4I View FIGURE 4 ). Short, length ca. 0.5 × CL, ca. 1.2 × sixth abdominal pleura length; broad, length ca. twice breadth; armed with 3–4 pairs of dorsal spiniform setae ( holotype with 2 spiniform setae on left side, 3 on right side), usually subequal in size, generally located in posterior half of telson, posterior pair sometimes situated close to posterior telson margin; posterior margin of telson rounded, without medial projection, bearing 1 pair of small lateral spiniform setae, 1 pair of much longer, sublateral, spiniform, non-plumose setae and 8–12 equally spaced, intermediate, spiniform, plumose setae, intermediate plumose setae increasing in length medially, slightly thinner than sublateral setae, scattered simple setae above posterior telson margin.
Pre-anal plate ( Fig. 4H View FIGURE 4 ). Armed with well-developed pre-anal carina, apex setose, non-spinate.
Life history. Developed ova (with eye spots) large, 0.75–1.0 mm × 0.4–0.65 mm; few in number, 3–11 per brood ( Fig. 5A View FIGURE 5 ). Ovigerous females collected in May during the early dry season.
The large ova are similar in size to the ova of C. formosae Hung, Chan & Yu, 1993 (ova 1.04 × 0.68 mm), a species with highly suppressed, larval development and an almost fully formed, benthic hatchling ( Shy et al. 2001).
Colour. Translucent with rust brown chromatophores. Chromatophores either small and widely spaced giving speckled appearance ( Fig. 5B View FIGURE 5 ) or forming large blotches ( Fig. 5A View FIGURE 5 ), blotches tending to be grouped as transverse bands on abdomen and posterodorsal carapace, widely spaced on ventral carapace and pereiopods, closely spaced on anterodorsal carapace. Ova rust brown ( Fig. 5A View FIGURE 5 ).
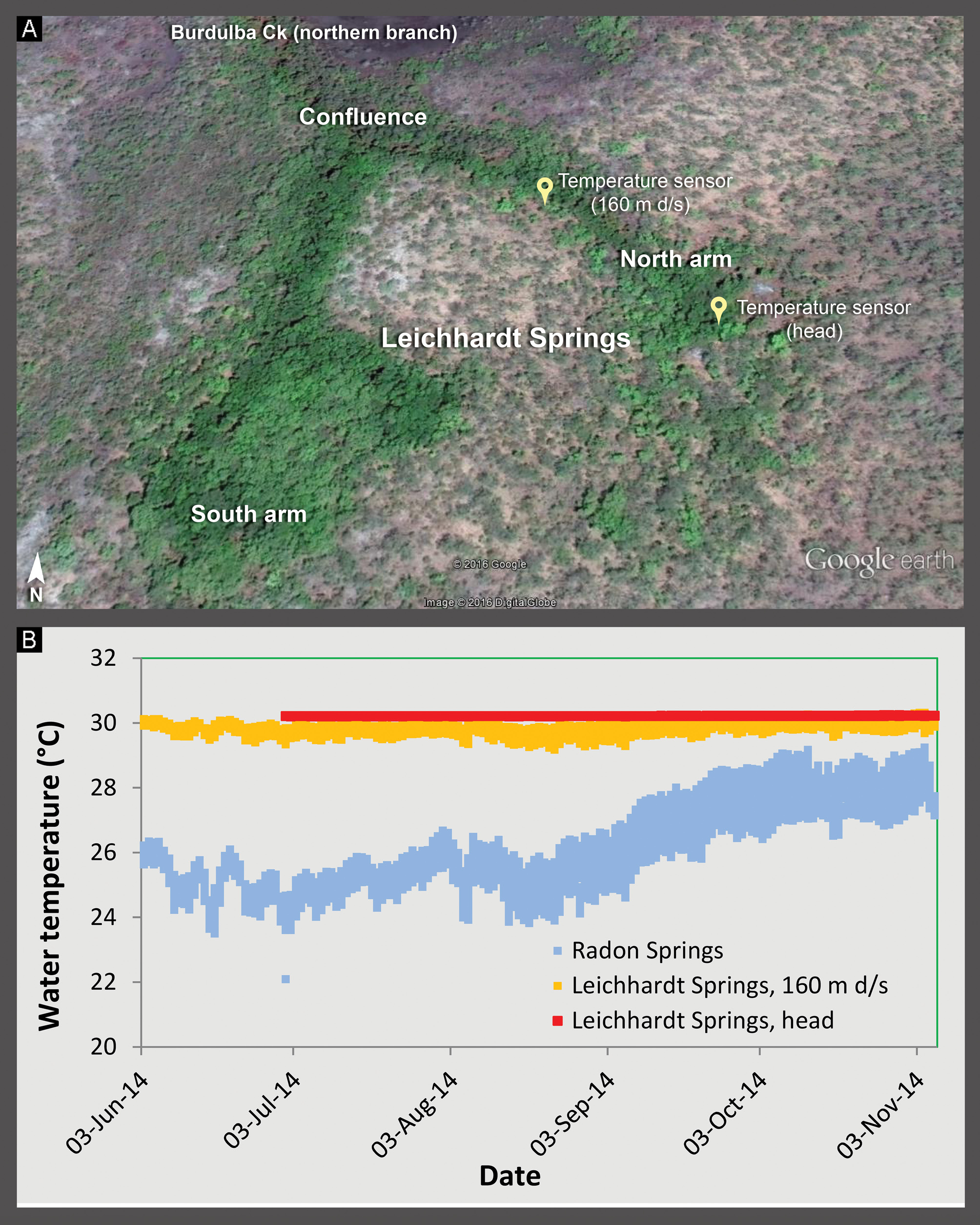
Habitat. Leichhardt Springs has two dominant outflows approx. 320 m apart—a north arm and a south arm, with a downstream confluence into the northern branch of Burdulba Creek ( Fig. 6A View FIGURE 6 ). The new species has so far been collected from both the north arm and south arm as far downstream as the junction between the two streams. The total length of watercourse in which the species is presently known is approx. 700 m.
The heat source of Leichhardt Springs is presently undetermined although other thermal springs in the Alligator Rivers Region (including western Arnhem Land) are known to be associated with high heat-producing rocks in the basement of the crust (S. Marshall, Geosciences Australia, pers. comm.). No other thermal springs are presently known from Kakadu NP.
The thermal properties of Leichhardt Springs were investigated over a four to five-month period during the 2014 winter-spring dry season using two CTD-Diver multi-parameter dataloggers deployed on the north arm of Leichhardt Springs, one at the head of the spring and the other 160 m downstream ( Figs. 6 View FIGURE 6 A–B). A third Diver was also deployed in Radon Springs, a non-thermal spring located approx. 6 km to the northeast on the large, outlying, sandstone formation above Leichhardt Springs ( Fig. 1 View FIGURE 1 ). Over the five-month period, water temperature at the head of the north arm spring was an invariant 30.2°C (SD = 0.005) and 160 m downstream, almost constant and only slightly cooler (mean 29.8°C, range 29.2–30.3°C). By comparison, Radon Springs showed a much higher temperature range (mean 26.0°C, range 22.1–29.3°C), thus 1–8°C cooler over the same period ( Fig. 6B View FIGURE 6 ). A sampling trip to the south arm of Leichhardt Springs in June 2016 revealed thermal properties similar to the north arm.
Apart from its thermal properties, the location of Leichhardt Springs relative to the Kakadu-Arnhem Land plateau and escarpment complex is also interesting. Other sandstone-formation springs in Kakadu NP arise within small gorges and typically at the immediate base of escarpments. Leichhardt Springs is located approx. 900 m away from the western escarpment of an outlying sandstone formation and at a lower elevation ( Fig. 5D View FIGURE 5 ).
Satellite imagery of Leichhardt Springs shows extensive rainforest vegetation along both arms associated with the permanent, spring-fed outflows ( Fig. 6A View FIGURE 6 ). The head of the north arm outflows through a patch of small, abraded and bleached, sandstone cobbles. For at least 300 m downstream, the north arm is characterised by a sequence of pools with clean, quartzite sand substrate interspersed with swift-flowing runs and riffles of gravel and cobble ( Fig. 5E View FIGURE 5 ). Riparian trees include Melaleuca spp., Carallia brachiata , Pandanus aquaticus , Canarium australianum , Syzygium spp. and the monsoon rainforest-specific species, Nauclea orientalis and Melicope elleryana . The understorey comprises climbing vines ( Flagellaria indica and Rhaphidophora australasica ), climbing ferns ( Stenochlaena palustris ), groundstorey ferns ( Blechnum orientale ) and the flowering shrub, Melastoma sp. The macrophyte, Eleocharis sp., is commonly interspersed amongst runs and riffles of the stream.
The new species is abundant amongst leaf litter and the fibrous roots of riparian trees and ferns along the edges of the stream. No shrimps were found in the clear, faster moving water in the runs and riffles.
The downstream distribution of the shrimps beyond the confluence between the north and south arm is presently unknown as is the dependence of the shrimps on constantly warm, spring waters.
Apart from water temperature, water quality of Leichhardt Springs is otherwise typical of surface waters draining the highly leached, sandstone formations of Kakadu NP and western Arnhem Land ( Short et al. 2013) being high in clarity, acidic, very soft and low in solutes. Key physicochemical variables at Leichhardt Springs were measured on 14 May 2003 and were as follows: turbidity <1 NTU; pH 5.2; electrical conductivity 16 µS cm–1; Ca and K < 0.1 mg L–1; Na 0.9 mg L–1; Mg 0.3 mg L–1; and SO 4 0.2 mg L–1.
Distribution. Endemic to Leichhardt Springs, Kakadu NP, Northern Territory, Australia. The known distributional range within the watercourse and associated riparian zones of Leichhardt Springs is 0.05 km 2.
Molecular results. The five specimens of Caridina biyiga sp. nov. sequenced for 16S produced two very closely related haplotypes of 539 bp. The single individual sequenced for the three remaining genes produced the following sequences: 3’ COI (557 bp), 5’ COI (629 bp) and 28S (500 bp).
A BLASTN comparison of GenBank sequences resulted in the following closest matches to the new species; 16S: Caridina thermophila —95% similar, C. ‘sp. WA3’—94%, C. ‘sp. NT1’—94%; 3’ COI: C. ‘sp. NT1’—90%, C. thermophila —87%, C. ‘sp. WA3’—87%, C. ‘sp. WA2’—86%; 5’ COI: C. ‘ sp. 3 Solomon’ - 85%, C. similis - 85%, C. simoni - 85%, C. ‘indistincta sp. A’ - 84%; 28S: C. similis - 85%, C. appendiculata - 85%, C. gracilipes - 85%. The closest matches of the 5’ COI sequence on the BOLD database were the same as those of the relevant GenBank results.
The best-fit models of molecular evolution for the molecular datasets were Hasegawa-Kishino-Yano with a gamma shape parameter and an estimated fraction of invariant sites for the 16S dataset, and Tamura 3-parameter with an estimated fraction of invariant sites for the 3’ COI dataset. Bayesian and maximum likelihood trees were produced for both datasets and a Bayesian tree for a small combined 16S/3’ COI dataset.
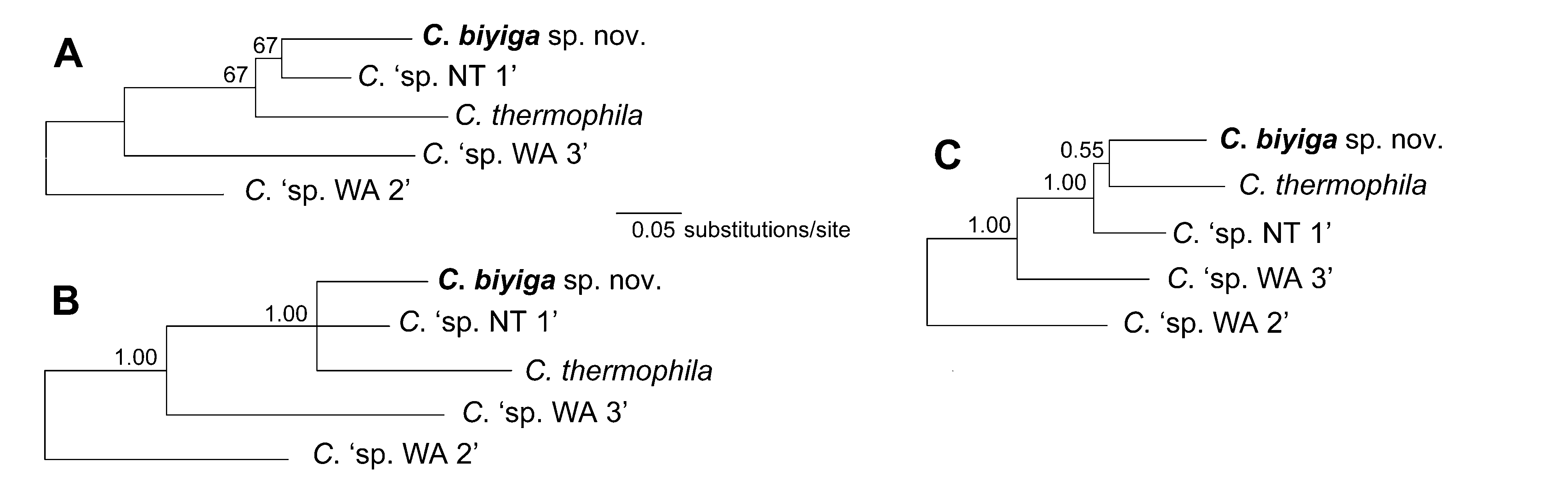
The topologies of both the Bayesian and maximum likelihood trees for the full 16S dataset (Bayesian arithmetric mean = –5050.72, maximum likelihood: log score = –4829.33) are very similar, in particular relating to the position of the new species ( Figs. 7–8 View FIGURE 7 View FIGURE 8 ). Both analyses recover C. ‘sp. WA3’ (Pilbara, WA), C. thermophila Riek, 1953 (central western Qld), and C. ‘sp. NT1’ (Melville Island, NT) as forming a well-supported clade with C. biyiga sp. nov. (Bayesian posterior probability 1.00; maximum likelihood bootstrap 92%), a ‘thermophila’ species group. Analyses of the large 16S dataset ( Figs. 7–8 View FIGURE 7 View FIGURE 8 ) are not clear on the relationships within the ‘thermophila’ group, whereas the 3’ COI (Bayesian = –1584.72, maximum likelihood: –1580.05) ( Figs. 9 View FIGURE 9 A–B) and combined 16S/3’ COI (Bayesian = –2538.83) data ( Fig. 9C View FIGURE 9 ) suggest C. ‘sp. WA3’ is sister to the other species in the group. The Kimura 2-parameter (K2P) genetic distances between C. biyiga sp. nov. and other species within the group ranged from 5.1–6.0% for the 16S dataset (closest C. thermophila ) and 10.4–15.1% for the 3’ COI dataset (closest C. ‘sp. NT1’).
The most closely related clade to the ‘thermophila’ group may be a moderately supported grouping of species all from the Kimberley, WA (Bayesian posterior probability 0.90; not supported by the maximum likelihood analysis). Within this clade, a grouping comprising C. spelunca Choy, 1996 ; C. ‘sp. WA2’; C. ‘sp. WA5’; and C. ‘sp. WA6’ was moderately supported by both the Bayesian and maximum likelihood analyses (Bayesian posterior probability 0.66; maximum likelihood bootstrap 74%) and is hereby referred to as the ‘spelunca’ group. K2P genetic distances between 16S sequences of C. biyiga sp. nov. and species from the ‘spelunca’ group ranged from 7.7–9.0% with a 3’ COI distance of 15.9% to C. ‘sp. WA2’ from that group.
Relationships of the remaining Australian Caridina species were partially resolved using the 16S dataset. Two species groups, which were also easily recognised using morphological characters, were recovered as strongly supported clades in both the Bayesian and maximum likelihood analyses; viz. a ‘gracilirostris’ group (Bayesian posterior probability 1.00; maximum likelihood bootstrap 99%) and a ‘serratirostris’ group (Bayesian posterior probability 1.00; maximum likelihood bootstrap 95%).
The Caridina zebra species complex (sensu Choy et al. 2019) also formed a strongly supported clade in both the Bayesian and maximum likelihood analyses (Bayesian posterior probability 0.99; maximum likelihood bootstrap 86%). This complex comprises three, highly similar species from the Tablelands and Cardwell Range areas of the wet tropics, viz. C. zebra Short, 1993 , C. confusa Choy & Marshall, 1997 and a recently described species, C. malanda Choy, Page, de Mazancourt & Mos, 2019 .
Another strongly supported clade included the species C. ‘sp. Gulf2’ from northwest Qld and C. ‘sp. NT2’ from the Darwin area, Northern Territory (Bayesian posterior probability 1.0; maximum likelihood bootstrap 100%), although the comparative morphology of the two species was not investigated in the present study.
Two representatives of the Caridina ‘sp. D’ complex, C. ‘sp. D’ and C. ‘sp. DG’, were recovered as a well-supported clade (1.00 Bayesian posterior probability; 100% maximum likelihood bootstrap). This species complex, which requires further study to resolve relationships between putative species, shows an extensive distribution across much of northern, eastern and central Australia. Material from east Queensland has previously been reported as C. nilotica aruensis (J. Roux 1926; Riek 1953; Glaister 1976) although the genetic relationship between Australian material and C. aruensis J. Roux, 1911 from the Aru Islands, Indonesia, is presently undetermined.
Two moderately to strongly supported clades containing species of Caridina as well as representatives of hypogeal genera were also recovered. The first of these clades (Bayesian posterior probability 0.97; maximum likelihood bootstrap 69%) comprised Caridina wilkinsi (Calman) , a wide-ranging, northern Australian species, with the eastern and central Australia species, C. ‘indistincta sp. A’, C. ‘indistincta sp. B’, C. ‘indistincta sp. C1’, C. ‘indistincta sp. C4’, C. ‘sp. LE’ and the northwest Australian, hypogeal species, Parisia gracilis Williams, 1964 (NT) and Pycneus morsitans Holthuis, 1986 (WA). The second clade (Bayesian posterior probability 0.83; maximum likelihood bootstrap 97%) included species of the Caridina zebra complex, C. spinula and the NT subterranean species, Parisia unguis Williams, 1964 and Pycnisia raptor Bruce, 1992 . Although presently not supported by morphological data, most of these relationships were also reported in the 16S analyses of Page et al. (2008b).
Within the two hypogeal-epigeal species clades reported above, strong relationships between P. gracilis and Pycneus morsitans (Bayesian posterior probability 1.0; maximum likelihood bootstrap 87%) and P. unguis with Pycnisia raptor (Bayesian posterior probability 1.0; maximum likelihood bootstrap 100%) were also recorded. These relationships have previously been reported in the 16S analyses of Page et al. (2008b) and von Rintelen et al. (2012). The close relationship of P. gracilis and Pycneus morsitans was also confirmed in the combined 16S/28S/H3 and 16S/28S analyses of von Rintelen et al. (2012). The molecular data strongly suggest that Australian Parisia are non-monophyletic and that the current generic classification of Australian species of Parisia , Pycneus and Pycnisia requires re-appraisal.
Systematic Position. Although the general morphology of the new species is typical of Caridina , the branchial formula, fingertips of the chelae and fifth pereiopod dactylus are unusual for the genus.
The new species lacks an arthrobranch at the base of the first cheliped and has 8 pairs of branchiae rather than the full complement of 9 branchiae normally associated with Caridina . There is also a vestigial epipod on the first maxilliped ( Fig. 3F View FIGURE 3 ) in addition to the epipods on maxillipeds 2–3 ( Figs. 3D, G View FIGURE 3 ) and pereiopods 1–4 ( Fig. 4J View FIGURE 4 ) typical of Caridina . Under the subfamilial and generic classification of Holthuis (1993), these differences would exclude the new species from Caridina and would assign the species to a different subfamily (Caridellinae rather than Atyinae ). However, recent studies ( von Rintelen et al. 2008; von Rintelen et al. 2012; De Grave & Page 2014) indicate that the branchial formula is less conservative within the Atyidae than previously accepted and minor differences in the development of the branchiae are not reliable for defining genera and subfamilies. For example, Caridinides wilkinsi Calman , which was recently transferred to Caridina by De Grave & Page (2014), has an exopod on the first pereiopod and a small epipod on the first maxilliped. Similarly, Cai & Shokita (2006) noted that the arthrobranch at the base of the first cheliped is sometimes reduced in C. serratirostris , and invariably absent in C. celebensis De Man, 1892 . In C. thomasi von Rintelen, Karge & Klotz, 2008 , there is only one arthrobranch rather than the usual two arthrobranchs at the base of the third maxillipeds. The branchial formula for C. biyiga sp. nov. falls within the wide range of variation now reported for Caridina sensu lato.
The fifth pereiopod dactylus of the new species is also atypical for the genus and is similar in shape to the dactyli of the third and fourth pereiopods ( Figs. 4 View FIGURE 4 E–F, K). In other species of Caridina , the fifth pereiopod dactylus is usually more slender and elongate than the preceding ambulatory pereiopods, as illustrated by Cai et al. (2006; Figs. 13F–I, 14E–H, 15G–J) for the type species, C. typus. In the new species, the number of spiniform setae on the flexor margin of the dactylus is also similar to the preceding two pereiopods. At most, there are four, widely spaced, claw-like spiniform setae on the flexor margin ( Fig. 4K View FIGURE 4 ). In other Caridina species, including C. typus (see Cai et al. 2006; Figs. 13I, 14H, 15J), there is a comb-like row of numerous, closely spaced setae.
In the new species, the fingers of the first and second chelae are distinctive for the genus in having flexible, spatulate tips ( Fig. 2H View FIGURE 2 ). In the type species of the genus, C. typus, the fingertips bear a rigid, hooked, nail-like unguis as illustrated by Bouvier (1925; Fig. 282).
Within the described Australian Caridina fauna, the new species is easily recognised by the short, slen- der, depressed rostrum bearing 3–7 longitudinally compressed, preorbital, dorsal teeth but lacking ventral teeth ( Figs. 2A, E View FIGURE 2 , I–J); the anteromedially produced scaphocerite lamina ( Fig. 2F View FIGURE 2 ); and the relatively short, first and second chelae which have length/breadth ratios less than 1.5 and 2.0 respectively ( Figs. 2 View FIGURE 2 C–D).
In regard to the short, slender, depressed rostrum, the new species shows some resemblance to C. confusa , C. malanda , C. spinula , and C. zebra from northeast Qld, but differs in having 3 or more dorsal teeth ( Figs. 2A, E View FIGURE 2 , I–J). It also differs in having the scaphocerite lamina anteromedially produced vs. anteromesially produced; 4 or fewer spiniform setae on the flexor margin of P5 dactylus vs. 45 or more spiniform setae on the flexor margin of P5 and in lacking an arthrobranch at the base of the first chelipeds.
Although the 16S molecular analyses placed the new species in a strongly supported clade, the ‘thermophila’ species group, these relationships are not readily apparent using morphological characters. In addition to the characters mentioned above for distinguishing the new species from its Australian congeners in general, the new species can easily be distinguished from C. thermophila and C. ‘sp. NT1’ within the ‘thermophila’ group, by the following characters:
1. rostrum depressed, with 7 or fewer dorsal teeth and lacking postorbital teeth ( Figs. 2A, E View FIGURE 2 , I–J) vs. a laterally compressed rostrum with 12 or more dorsal teeth including 1–4 postorbital teeth;
2. antennal spine fused with, and indistinct from, inferior orbit ( Figs. 2A View FIGURE 2 , I–J) vs. antennal spine situated below and distinct from inferior orbit in C. thermophila and C. ‘sp. NT1’ (Fig. 14B);
3. flexor margin of P5 dactylus with 3–4, widely spaced, spiniform setae ( Fig. 4K View FIGURE 4 ) vs. a comb-like row of 45–49 closely spaced, spiniform setae on the flexor margin of P 5 in C. thermophila and a similar row of ca. 40 closely spaced, spiniform setae in C. ‘sp. NT1’;
4. flexor margin of P3 dactylus bearing 3–5 spiniform setae ( Fig. 4E View FIGURE 4 ) vs. 8–11 spiniform setae in C. thermophila and ca. 7 spiniform setae in C. ‘sp. NT1’.
Recent COI meta-analyses for the Decapoda are helpful to some degree regarding genetic evidence for or against the recognition of a new genus to accommodate the new species. The closest sister taxon to the new species based on the 3’ COI fragment was C. ‘sp. NT1’ in the ‘thermophila’ species group with a K2P distance of 10.4%. This is slightly below the 11.27–49.93% range of genetic difference between genera within a family reported by Costa et al. (2007) and significantly lower than the 19.75% average they reported. It is also lower than the 22.33% average reported by Matzen da Silva et al. (2011) for decapods but falls within the large overall range between genera of 6.69–48.35%.
Although both the genetic and morphological data indicate that C. biyiga sp. nov. is a highly distinctive species within the Australian Caridina fauna, there does not appear to be sufficient evidence at this point to warrant the recognition of a new monotypic genus. The genetic analyses placed the new species in the ‘thermophila’ group, a strongly supported clade including at least one typical species of Caridina , viz. C. thermophila . The K2P genetic distances between the new species and other species within the clade are also towards the lower end of the range recorded between genera in decapod meta-analyses ( Costa et al. 2007; Matzen da Silva et al. 2011). We therefore assign the new species to the genus Caridina .
The following illustrated key can be used to identify the new species from other Northern Territory Caridina . The inclusion of C. typus in the key is based solely on the Australian distribution given by Horwitz (1995), ‘ Northeastern Queensland to Northern Territory’ , which is possibly erroneous. Although the species is known from eastern Queensland , we have so far been unable to find other records or material of the species from the Northern Territory.
TABLE 3. Branchial formula of Caridina biyiga sp. nov.
| Maxillipeds | Pereiopods | |||||||
|---|---|---|---|---|---|---|---|---|
| I | II | III | 1 | 2 | 3 | 4 | 5 | |
| Pleurobranchs | – | – | – | 1 | 1 | 1 | 1 | 1 |
| Arthrobranchs | – | – | 2 | – | – | – | – | – |
| Podobranchs | – | 1 | – | – | – | – | – | – |
| Epipods | 1 | 1 | 1 | 1 | 1 | 1 | 1 | – |
| Exopods | 1 | 1 | 1 | – | – | – | – | – |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |