Semnopithecus entellus (Dufresne, 1797)
|
publication ID |
https://doi.org/10.5281/zenodo.6867065 |
|
DOI |
https://doi.org/10.5281/zenodo.6863436 |
|
persistent identifier |
https://treatment.plazi.org/id/CE199B17-FFA8-FFAE-FF36-64A3FD6CFD7C |
|
treatment provided by |
Jonas |
|
scientific name |
Semnopithecus entellus |
| status |
|
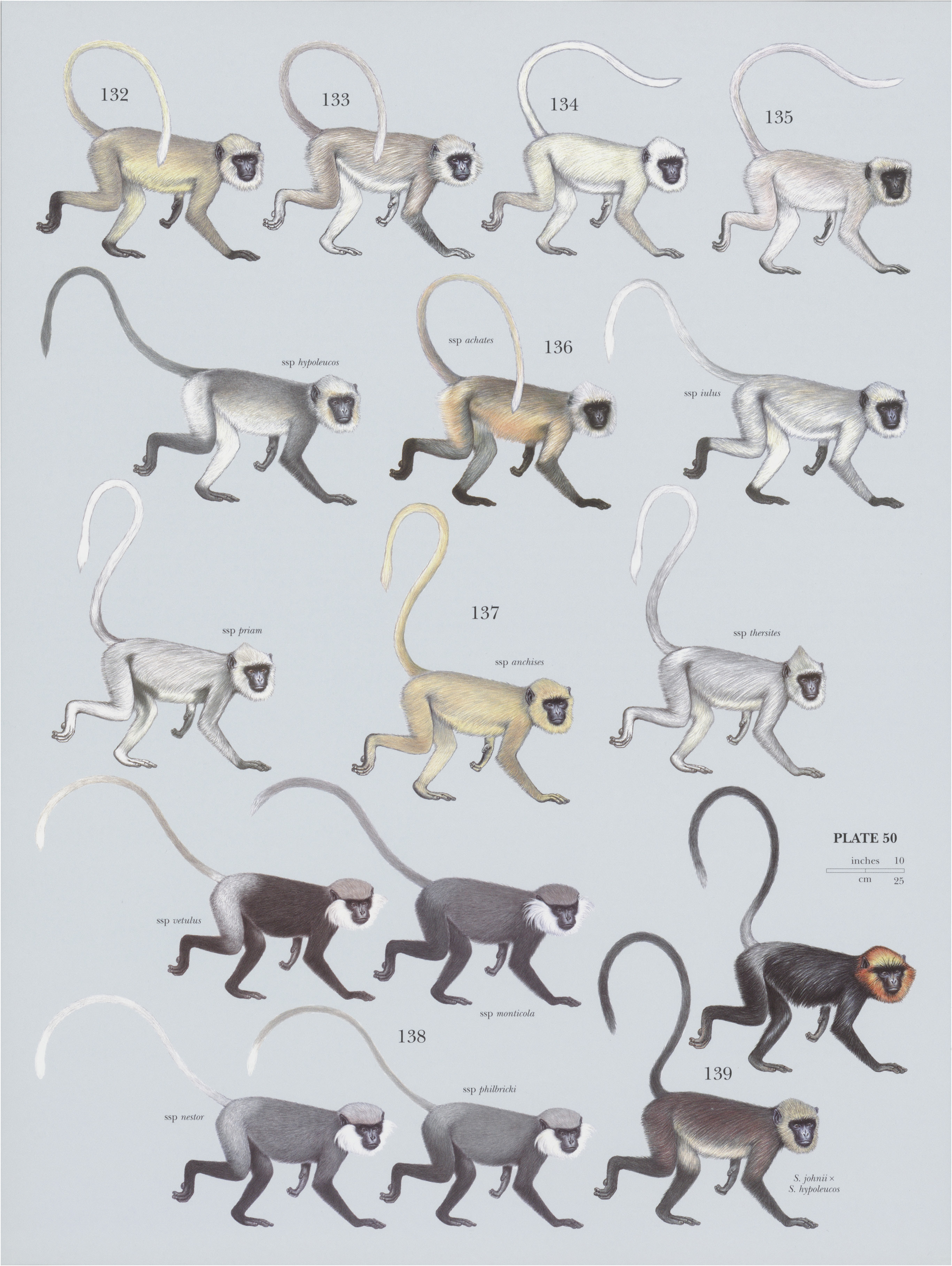
132. View Plate 50: Cercopithecidae
Bengal Sacred Langur
Semnopithecus entellus View in CoL
French: Langur du Bengale / German: Bengalen-Hulman / Spanish: Langur de Januman
Other common names: Bengal Gray Langur, Bengal Hanuman Langur, Common Langur, Entellus Langur, Hanuman Langur, North Indian Langur, Northern Plains Gray Langur
Taxonomy. Simia entellus Dufresne, 1797 ,
India, Bengal.
S. entellus shows considerable variation in size and coat color throughout its distribution. For many years, the Hanuman or gray langurs of South Asia were all classified as subspecies of S. entellus , but W. C. O. Hill in his 1939 review and C. P. Groves in his 2001 Primate Taxonomy considered it to be monotypic, and this has been confirmed with recent genetic studies. M. L. Roonwal separated gray langurs of South Asia into a northern group and a southern group based on tail carriage. S. entellus is of the northern group (Type IA), with the tail looping forward and dangling down and the tip reaching back, even hanging down on one side of the body. Monotypic.
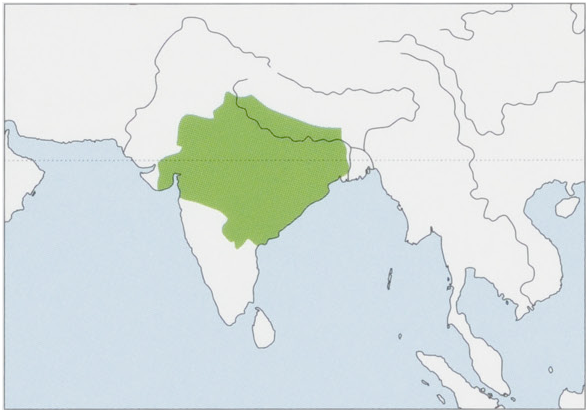
Distribution. India, the species’ range extends 1900 km W to E, and includes all populations with the northern-type tail carriage between the Tapti River in Gujarat State and Krishna River in Andhra Pradesh State to the foothills of the Himalaya. Introduced into SW Bangladesh, where the population may have arisen from descendents of a pair introduced by Hindu pilgrims on the banks of the Jalangi River. View Figure
Descriptive notes. Head—body 45.1-78.4 cm, tail 80.3-111.8 cm; weight 16.9-19.5 kg (males) and 9.5-16.1 kg (females). Coat of the Bengal Sacred Langur has short, wavy hairs that are creamy-yellow on head and flanks and brown on back and limbs. Tail has a white tip. Underside is red-gold. Cheek whiskers are long and whitish. Face and ears are black. Eyebrows are long and bristle-like. Crown has no tuft, and hairs are parted neatly along midline. Hands and feet are black, in contrast to brownish limbs.
Habitat. Mostly dry tropical deciduous forest, tropical thorn forest in parts of Gujarat and Rajasthan; tropical moist deciduous forest on the eastern and south-eastern edges of its distribution; and human-modified sparsely wooded areas and scrublands. Bengal Sacred Langurs are often found in proximity to human habitation. They are a lowland plains species; their ranges extend up to elevations of 400 m.
Food and Feeding. Bengal Sacred Langurs feed on leaves, shoots, fruits, buds, flowers, tree exudates, bark, vines, grasses, galls and insect larvae, and termite soil. At Kanha, Madhya Pradesh, the annual diet of a group (time spent feeding on different food items) was 39% leaves (mostly mature 35%; young leaves 4%), 24% seeds and fruits, 9% flowers, 11% buds and bark, and 3% other items including insects. Caterpillars and other insects can make up to 25% of their diet in monsoon months. No single species predominated throughout the year. The group exploited 53 species of trees and vines. Young leaves were eaten in preference to mature leaves, and fruits and flowers in preference to leaves. The ability of Bengal Sacred Langurs to survive on diets of largely mature leaves is fundamental to their survival in dry seasons when canopies of deciduous forests become leafless and only understories remain green. They play an important role in seed dispersal of trees such as Aglaia lawii ( Meliaceae ). They feed on bark of Boswellia serrata ( Burseraceae ), Ficus glomerata ( Moraceae ), Anogeissus latifolia ( Combretaceae ), Albizia odoratissima ( Fabaceae ), and Tamarindus indica (Fabaceae) , especially during dry seasons when food is limited. They also feed on cultivated crops.
Breeding. Breeding and mating of the Bengal Sacred Langur occur throughout the year when food is abundant, and especially when they are provisioned in temples, urban areas, or villages, or have access to crops. In forest groups and more seasonal areas, the birth season is in December—May. Seasonality in conceptions is sometimes apparent, but this is an effect of varying food availability. Only females in sufficient physical condition conceive. Males and females reach puberty at c.4 years of age but are not fully developed until 6-7 years. The menstrual cycle is 25-27 days. Females show proceptive behavior; shaking their heads, hopping, lowering their tails, and presenting their hindquarters in front of males. The gestation period is ¢.200 days (189-208 days), and the interbirth interval is ¢.16-7 months. At Jodhpur, Rajasthan, females have their first infant when they are c.3-5—4 years old. Infants are usually born single. A mother suckles her infant for 12-13 months. Females other than the mother carry and care for infants (alloparenting) for their first month or so. From birth to ¢.5 months, infants are brown, but from then to c.12-15 months, their pelage changes to a pale off-white and then to that of adults. Males are considered adult at 6-7 years old. Bisexual groups atJodhpur and Abu are not closed in terms of breeding. All-male groups are frequently near mixed groups and often able to associate closely with receptive/proceptive females. While rapid all-male replacement and subsequent infanticide (to make females return to ovulatory cycling and sexual receptivity) by groups of males happens at Abu and Jodphur, male replacement also occurs as a gradual process over 2-3 months, without takeovers and infanticide. Temporary associations of non-group adult males and females in bisexual groups are more common than changes in male membership involving actual replacement. As is typical of colobines, resident males are passively tolerant of infants but defend them when they are threatened. At Orcha, Madhyar Pradesh in the south-western part of their distribution, male Bengal Sacred Langurs are indifferent to male infants until they are ¢.10 months old. A male infant at this age will run up to a passing male, mountit, and then, as its dismounts, the male sits and the infant runs round to face him and they embrace. This behavior is displayed by young males until they are c.4 years old.
Activity patterns. Bengal Sacred Langurs are diurnal and primarily terrestrial. The activity budget of a group at the Kanha Tiger Reserve, Madhya Pradesh, was 41-8% inactive (resting), 25-7% feeding, 13-1% traveling, and 19-4% engaging in social and other activities. When fleeing, they tend to run along the ground rather than through trees. During a total eclipse, Bengal Sacred Langurs go into a state of total dormancy and inactivity, regaining normality only when the eclipse is over.
Movements, Home range and Social organization. Bengal Sacred Langurs live in one-male or multimale bisexual groups and all-male groups. Group sizes are usually 30-80 individuals. At the Sariska Wildlife Refuge in Rajasthan, they are 30-125 (average 64), and the male-to-female sex ratio is 1:2-6. At the Kanha Tiger Reserve, unimale—multifemale groups can be as large as 34 individuals, with up to 15 adult females. Most males there live in unimale—multifemale groups, with an average of 22 individuals. In Jodhpur, all males are either solitary, live in unimale-multifemale, or all-male groups; average size is 38 individuals, with an adult sex ratio of 1:4-9. At Orcha, maleslive in either multimale-multifemale or all-male groups, but not in unimale-multifemale groups; average size is 19 individuals, with an adult sex ratio 1:1-6. Large groups are associated with more open habitats, their degree of terrestriality, and increased threats from ground predators such as Leopards (Panthera pardus) or Tigers (P. tugris). When not feeding, males, not females, tend to sit up in trees, vigilant for other langur groups and predators. Related to this, Chital (Axis axis) are often found in association with Bengal Sacred Langurs. Chital exploit the vigilance of male langurs, while langurs undoubtedly benefit from the enhanced sense of smell and hearing in the Chital. They respond to each others’ alarm calls. In these large groups, males show no strict dominance hierarchy and are quite tolerant of each other. Females are philopatric, they stay in the group they were born in, but males disperse and become temporarily solitary or join up with all-male groups. Solitary males are healthier than males living in groups. Females groom males and other females, but males very rarely groom females. Females have a strict dominance hierarchy among themselves that is influenced by age. Younger females are higher ranking, and they give and receive more grooming than older females and those lower in the hierarchy. Females in conflict show a curious behavior ofsitting erect, face-to-face, while slapping each other with both hands. Male group sizes are 2-43 individuals, with a mean of 21. Home ranges are 40-90 ha. Home range sizes are influenced by temporal and spatial variation in abundance and dispersion of food sources but also by proximity to agricultural crops, food provisioning, and other environmental factors. Daily movement can be more than 1000 m. Bengal Sacred Langurs travel further when their diets are more focused on fruits and flowers than leaves. At least in some populations (Abu, Jodhpur, and Kanha), male takeovers (invasion of bisexual groups by nongroup males, eviction of resident group males, and annexation of the group’s females and immature members) are sometimes rapid and complete and associated with subsequent infanticide. In other populations (Kaukori, Sariska Wildlife Sanctuary, and Gir Wildlife Sanctuary), male membership of groups changes gradually, without involving abrupt takeovers and infanticide. At Kanha Tiger Reserve, male tenure as a dominant in a group averaged c.45 months. Males in unimale—multifemale groups at Jodhpur manage an average tenure of 2-2 years. Adult males may leave the group and live alone or join all-male groups. Bengal Sacred Langurs are skilled at raiding crops, with strategic raiding operations where a few individuals act as guards and the rest of the group feeds. Robust male members of unimale and multimale bisexual groups and all members of all-male groups are more frequent crop raiders than females. Females with infants enter fields to raid crops only when males are with them to act as guards. Densities of Bengal Sacred Langurs vary considerably in different populations. At Orcha, Madhya Pradesh, in the south-western part of their distribution, they can be as low as 5 ind/km?, but they reach 46 ind/km? in Kanha Tiger Reserve, north of Orcha, and 121 ind/km? in Gir Forest.
Status and Conservation. CITES Appendix I. Classified as Least Concern on The [UCN Red List. The Bengal Sacred Languris listed on Schedule II, Part I of the Indian Wildlife (Protection) Act, 1972, amended up to 2002, and Schedule III, Bangladesh Wild Life (Preservation) Order, 1973. The Bengal Sacred Langur occurs in many protected areas. Its status in captivity is difficult to determine because of taxonomic confusion with related forms. It is listed as Least Concern in view of its wide distribution, tolerance of a broad range of habitats, and presumed large population. If threats arising from hunting and human-animal conflicts increase (e.g. in Andhra Pradesh and Orissa), the Bengal Sacred Langur will need to be reassessed and could qualify for listing as Near Threatened. Its total population size is unknown. Most Bengal Sacred Langurs now occupy human-dominated landscapes, with very few actually occurring in forested areas. Habitat loss, intensive agriculture, forest fires, and conflict with humans (including road kills) are major threats. They like to stay and move close to roads or on them because roads are avoided by predators such as feral dogs and leopards. Bengal Sacred Langurs are revered and fed in temples.
Bibliography. Bennett & Davies (1994), Borries, Koenig & Winkler (2001), Borries, Sommer & Srivastava (1991, 1994), Chhangani (2002, 2004), Chhangani & Mohnot (2004, 2006), Dolhinow (1972), Groves (2001), Hill (1939), Hrdy (1974), Hrdy & Hrdy (1976), Karanth (2010), Karanth et al. (2010), Kirkpatrick (2011), Koenig et al. (1997), Lhota et al. (2001), McKenna (1978), Mohnot (1971a, 1971b), Molur et al. (2003), Murmu et al. (1998), Nag et al. (2011a, 2011b), Newton (1987, 1992), Newton & Dunbar (1994), Oppenheimer (1977), Punekar (2002), Rajpurohit & Mohnot (1988, 1991), Rajpurohit, Chhangani, Rajpurohit, Bhaker et al. (2008), Rajpurohit, Chhangani, Rajpurohit & Rajpurohit (2004), Roonwal (1984, 1986), Roonwal & Mohnot (1977), Roonwal, Prita & Saha (1984), Sharma (2001), Sommer et al. (1992), Sugiyama (1965a, 1965b, 1966), Sugiyama et al. (1965), Vogel (1971), Winkler et al. (1984), Yoshiba (1968).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Cercopithecinae |
|
Genus |
Semnopithecus entellus
| Russell A. Mittermeier, Anthony B. Rylands & Don E. Wilson 2013 |
Simia entellus
| Dufresne 1797 |