Pareuchiloglanis hupingshanensis, Kang & Chen & He, 2016
|
publication ID |
https://doi.org/10.11646/zootaxa.4083.1.6 |
|
publication LSID |
lsid:zoobank.org:pub:518BA705-C96A-47C0-BCA6-AB5966117285 |
|
DOI |
https://doi.org/10.5281/zenodo.5618327 |
|
persistent identifier |
https://treatment.plazi.org/id/CD0D9730-1760-1F0D-FF14-FD0A7D362AD6 |
|
treatment provided by |
Plazi |
|
scientific name |
Pareuchiloglanis hupingshanensis |
| status |
sp. nov. |
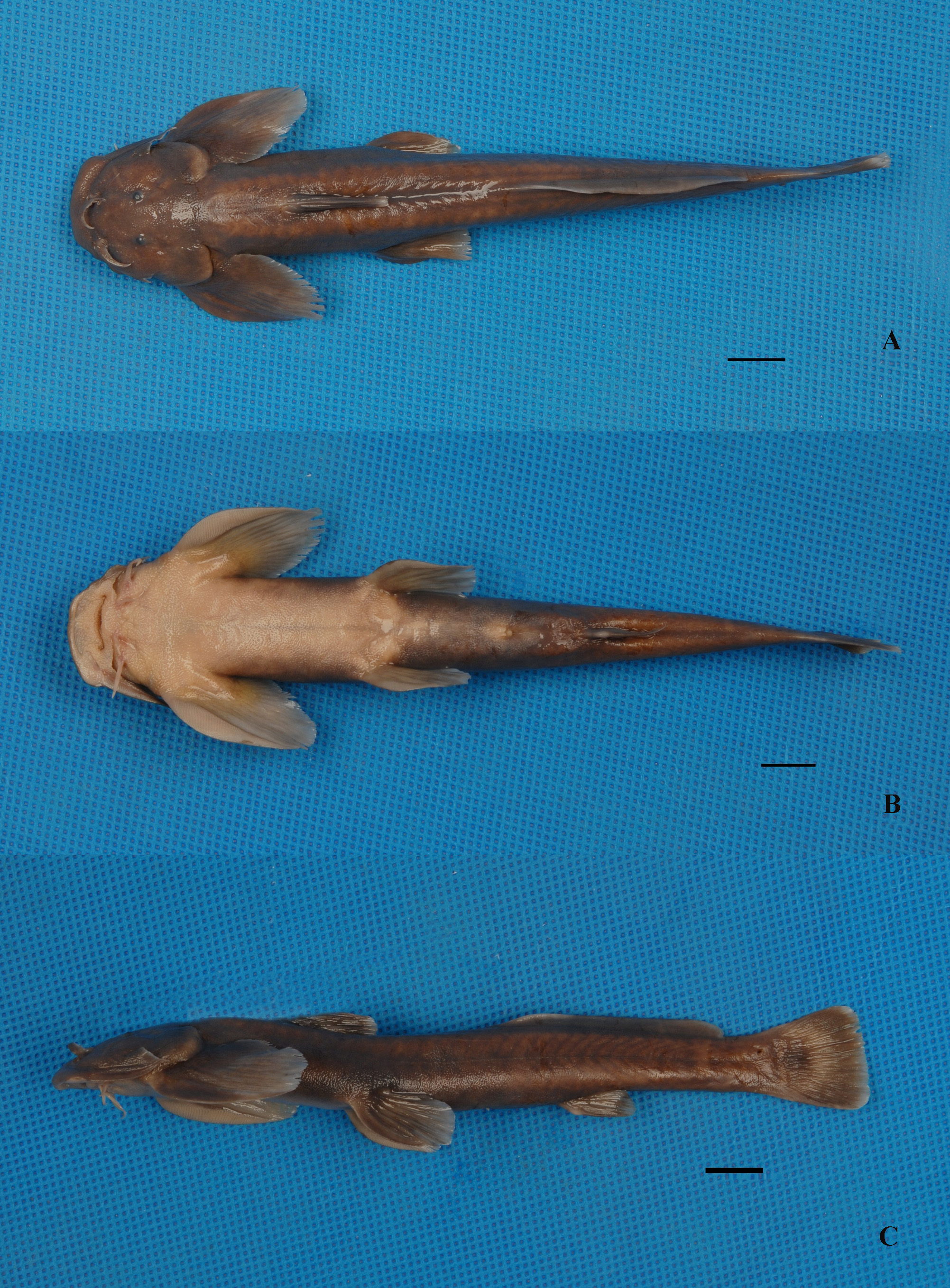
Pareuchiloglanis hupingshanensis , sp. nov.
( Fig. 3 View FIGURE 3 )
Holotype. HHNNR 20071104074 , adult male, 128.8 mm SL. Zhipeng River (upper Jiangping River, a tributary of Lishui River) , 3000'47.9̋ N 11033 View Materials '32.2̋E, Shimen County, Hunan Province, China, 4 November 2007, Z. Kang and J. Huang.
Paratypes. HHNNR 20070819425, 20071130392 (2, 133.3– 147.8 mm SL), Yangliu River, 2007, Z. Kang and J. Huang. HHNNR 20080622155, 20080622223, 2008062224 (3, 78.8–90.6 mm SL), Zhipeng River, 2008, Z. Kang and J. Huang. HHNNR 20080807516, 20080817005–06, 20080817008–09, 20080925019, (6, 88.8–154.2 mm SL), Yangliu River, Shangshenxi River and Zhipeng River, 2008, Z. Kang and J. Huang. HHNNR 20080906513–15 (3, 148.4– 157.9 mm SL), Shangshenxi River and Yangliu River, 2008, Z. Kang and J. Huang. HHNNR 20090905001–11 (11, 73.7–124.7mm SL), Shangshenxi River, 2009, Z. Kang and J. Huang. HHNNR 2010062109, 2010091912, 20100926014 (3, 75.9–141.4 mm SL), Yangliu River and Zhipeng River, 2010, Z. Kang and J. Huang.
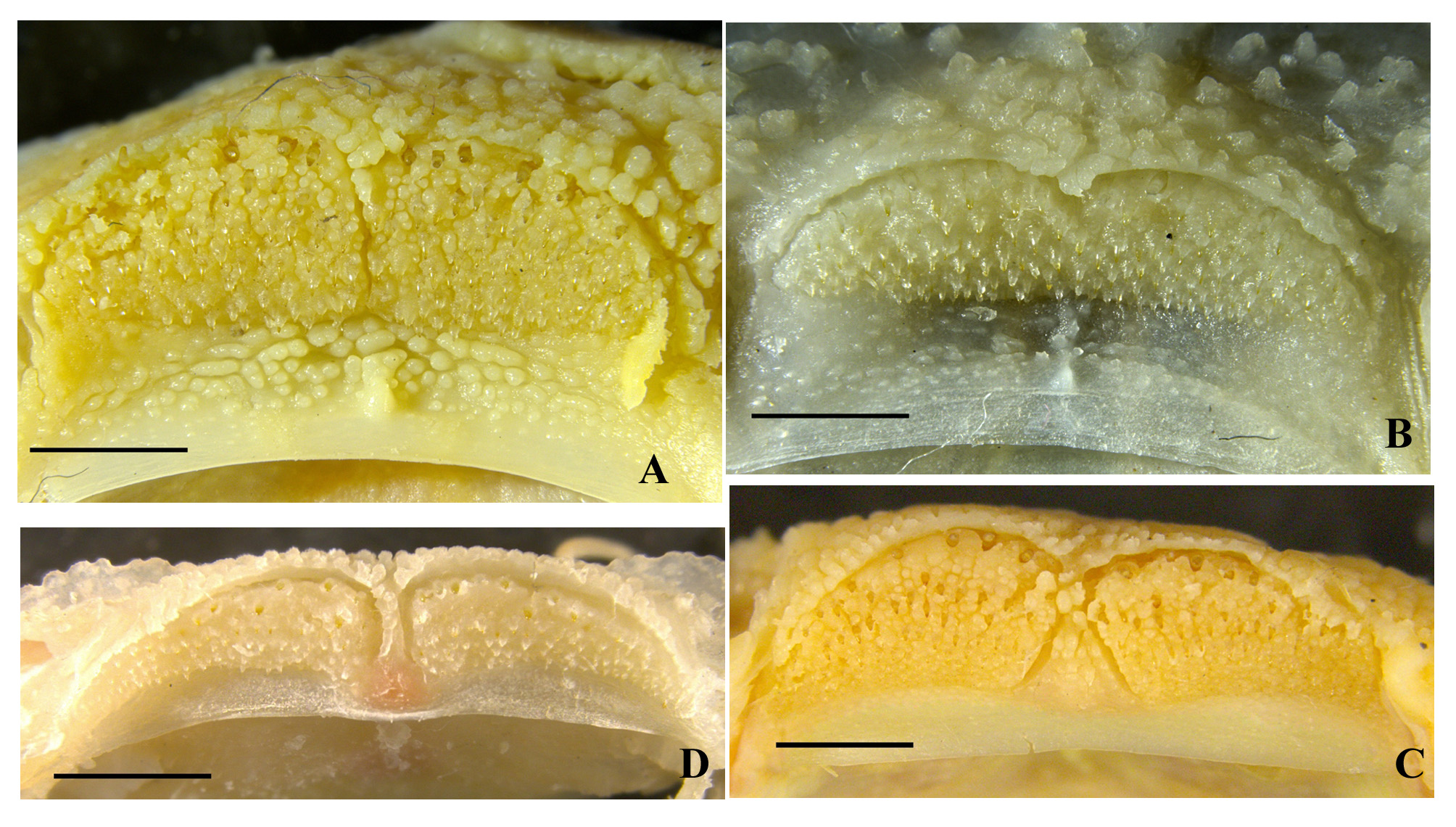
Diagnosis. Pareuchiloglansis hupingshanensis is distinguished from the other species of Pareuchiloglansis known from the upper Yangtze River in China ( P. anteanalis , P. sichuanensis , P. robustus , P. sinensis and P. tianquanensis ) in having a more restricted gill opening (reaching to the level of the fourth or fifth pectoral-fin element ventrally vs. not extending ventrally beyond the third pectoral-fin ray or above the middle of the pectoralfin base). It further differs from P. sichuanensis and P. tianquanensis in having the adipose fin separate from (vs. confluent with) the caudal fin, a greater caudal-peduncle depth (2.2–4.0 times in caudal peduncle length vs. 1.4– 2.1), the posterior end of pectoral-fin not reaching (vs. reaching) pelvic-fin origin, origin of anal fin midway between posterior end of pelvic-fin base and caudal-fin base (vs. nearer to anal-fin origin); from P. anteanalis in having a shorter pectoral fin (not reaching vs. reaching or exceeding the pelvic-fin origin); from P. anteanalis and P. robustus in having the origin of anus midway between posterior end of pelvic-fin base and anal-fin origin (vs. nearer to posterior end of pelvic-fin base). It is further differs from P. sinensis in having the premaxillary tooth band with a deep (vs. shallow) median indentation ( Fig. 4A–B View FIGURE 4 ), a dentary tooth band which consists of two short, wide (vs. long, narrow) patches ( Fig. 4C–D View FIGURE 4 ), edge of upper lip with dense (vs. sparse) papillae ( Fig. 5 View FIGURE 5 ), the posterior end of the ventral fin notably not reaching (vs. almost reaching) anus, a shorter dorsal fin, 56.7%–69.7% (mean 64.8%) HL (vs. 71.9%–86.1% (mean 76.5%) HL), a greater caudal-fin base depth, 35.2%–51.4% (mean 41.3%) HL (vs. 25.2%–35.2% (mean 29.4%) HL).
Description. Morphometric characters are given in Table 2. D. I-5-6, P. I-13-14, V. I- 5, A. I- 4, C. I-14-16 -I. Body long, compressed. Head wide, depressed, rostral margin rounded when viewed dorsally. Profile of head and occiput rising. Head and abdominal region moderately broad. Length of head 3.7 to 5.0, depth of body is 6.7 to 9.0 in standard length. Head slightly longer than broad. Mouth wide, inferior, transverse. Postlabial groove interrupted, ending at base of inner mandibular barbel. Oral region, anterior part of abdomen with dense papillae, density gradually decreasing posteriorly. Oral teeth conical, pointed, coniform, embedded in skin with only tips exposed, in irregular rows. Premaxillary tooth patches broad, appearing joined, with deep median indentation, laterally not extending backwards. Dentary tooth band divided into 2 fragments. Four pairs barbels, flattened. Nasal barbel with small flap of thin skin fringing posterior margin, reaching anterior margin of orbital. Maxillary barbel with thin flap of skin fringing posterior margin, with pointed tip, extending beyond lower end of gill opening. Two pairs of mandibular barbels, origin of inner mandibular barbel close to midline. Outer mandibular barbel originating posterolateral of inner mandibular barbel, notably short of origin of pectoral fin. Eye small, almost round, subcutaneous, located on upper lateral surface of head, closer to snout than to upper limit of gill opening. Lower lip connected to base of maxillary barbel by skin flap, without sulcus between them. Gill opening extending from posttemporal region to base of the 4th or 5th branched pectoral-fin ray.
Dorsal fin somewhat long, located in anterior third of body, without spine, its margin straight; tip of dorsal-fin rays extending beyond vertical through posterior end of pelvic-fin base. Origin of dorsal anterior to tip of adpressed pectoral fin. Dorsal-fin length slightly greater than body depth. Adipose fin located in half of postdorsal distance, posterior end of adipose fin base not confluent with caudal fin, base of adipose fin less than predorsal length. Paired fins broad, rounded, horizontally placed, first ray of each broadened; regular striae on ventral surface; posterior end of pectoral fin not reaching origin of ventral fin, posterior end of ventral fin not reaching anus. Pectoral fin with 13 branched rays, slightly shorter than head length. Pelvic fins with five branched rays, slightly longer than dorsal fin. Anus nearer to posterior end of pelvic-fin base than to anal-fin origin. Origin of anal fin inserted midway between origin of pelvic fin and caudal-fin base. Caudal fin truncate, about as long as anal fin. Skin smooth. Lateral line complete, midlateral.
Coloration. In fresh specimens, body greenish-yellow or light orangish-yellow on dorsal surface in summer, brown in autumn. Venter cream. A yellow spot on origin of dorsal and anal fin bases. Dorsal fin green-yellow with lighter medial band and distal edge. Adipose fin with ligh-yellow distal edge. Pectoral and pelvic fins greenish yellow with lighter color around distal edge. Caudal fin grayish black with small yellow patches in middle. Body grey in formaldehyde-fixed specimens. Color in spirit dark greyish. Ventral surface and free ends of fins lighter in color.
Distribution. Pareuchiloglanis hupingshanensis occurs in the Yangliuhe, Shangshenxihe, and Zhipenghe Rivers. All are tributaries of the Lishui River, in the middle reaches of the Yangtze River system.
Etymology. The species’ name, hupingshanensis refers to the type locality, Hupingshan.
Habitat and biology. This catfish inhabits fast-flowing rivers with sand and gravel substrate. Average absolute fecundity ranges from 196 to 297 eggs per gonad. The egg diameter ranges from 3.2 to 4.1 mm (N=3, HHNNR 2010062101, 2010062106, 2010062112, 150– 200 mm SL, Yangliu River, 2010, Z. Kang and J. Huang). Spawning occurs in June. Food consists mainly of caddisfy larvae (Trichoptera), Cyzicus spp. (Crustacea), larvae and adults of Luciola chinensis and Hydrophilid beetles ( Coleoptera ), and nymphs of dragonflies (Odonata) (N=5, HHNNR 2010062101, 2010062106–08, 2010062112,Yangliu River, 2010, Z. Kang and J. Huang).
Morphometry. The results of the principal components analysis (PCA) revealed a complete separation between P. hupingshanensis and the three species of Pareuchiloglanis ( P. anteanalis , P. robusta and P. sinensis ) along the third principal component (PC3) ( Fig. 6 View FIGURE 6 ). The first three principal components (PC1, PC2 and PC3) accounted for 72.8%, 10.3% and 5.5% of the total variance, respectively. Measurement loadings on PC3 are strongly related to caudal peduncle depth (-0.467), caudal-fin base depth (-0.350), eye diameter (-0.324), dorsal fin length (0.305), caudal fin length (0.250), pelvic fin length (0.223), distance between the origin of adipose fin and origin of anal fin (0.197), distance between the terminal of dorsal fin base and origin of adipose fin (-0.193), dorsal fin base length (0.192), distance between posterior insertion of adipose-fin base and ventral base of caudal fin (- 0.183), and pectoral fin length (0.182).
Characters Pareuchiloglanis hupingshanensis (n=29) P. sinensis (n=22) P. robusta (n=12)
……continued on the next page Characters Pareuchiloglanis hupingshanensis (n=29) P. sinensis (n=22) P. robusta (n=12) 0.32 0.24 3 component 0.16 0.08 Principal 0.00 -0.08
-0.16 -0.24 -0.4 -0.3 -0.2 -0.1 0.0 0.1 0.2 0.3 0.4 0.5
Principal component 2
The linear regression models were fitted by the dorsal-fin length, pectoral-fin length, pelvic-fin length and caudal-fin length against SL; and the caudal-fin base depth and eye diameter against HL for three species ( P. hupingshanensis , P. robusta and P. sinensis ), respectively. The significance test showed that the regression coefficients or intercepts were significantly different ( Fig. 7 View FIGURE 7 , Table 3) between P. hupingshanensis and the other two species, indicating that the difference is not due to ontogeny alone.
Sequence analysis of the cyt b gene. Only one haplotype of the mitochondrial cytochrome b (cyt b) gene (1 138 bp) was identified from eleven individuals of P. hupingshanensis in three localities, indicating a bottleneck effect due to the small population and restricted distribution. The mean number of pairwise differences between species of Pareuchiloglanis ranged from 0.23% (between P. sinensis and P. anteanalis ) to 12.8% (between P. sinensis and P. macrotrema ), and the sequence divergence levels of P. hupingshanensis with other species of Pareuchiloglanis ranged from 1.67% to 12.75% ( Table 4 View TABLE 4 ). On the whole, there is a low level of sequence divergence within Pareuchiloglanis . For example, two species, P. sinensis ( AY191609 View Materials ) and P. anteanalis (AY19161), share a single haplotype. Even betwee genera, P. feae Vinciguerra ( JN986971 View Materials ) and Creteuchiloglanis . gongshanensis Chu ( JN986970 View Materials ), also shared a single haplotype ( Fig. 8 View FIGURE 8 ). However, no haplotype was shared between P. hupingshanensis and other species of Pareuchiloglanis .
The ML tree which was recovered from the cyt b gene sequences is shown in Figure 8 View FIGURE 8 . The topology obtained from Bayesian inferences was similar to the ML tree. The monophyly of Pareuchiloglanis is not supported by the cyt b data. Four species from the Yangtze River, P. hupingshanensis , P. sinensis , P. anteanalis and E. kishinouyei , which are assigned to different genera, formed a clade with strong support (BP = 93, BBP = 1.00) ( Fig. 8 View FIGURE 8 ). However, the new species is distinctly separated from P. sinensis and P. anteanalis .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |