Saccharomyces cerevisiae
|
publication ID |
https://doi.org/10.1016/j.phytochem.2021.112954 |
|
DOI |
https://doi.org/10.5281/zenodo.8270342 |
|
persistent identifier |
https://treatment.plazi.org/id/C82A87F6-D445-F32B-FE23-FD92378FFBC8 |
|
treatment provided by |
Felipe |
|
scientific name |
Saccharomyces cerevisiae |
| status |
|
2.6. Functional characterization of Aa7DR 1 in S. cerevisiae
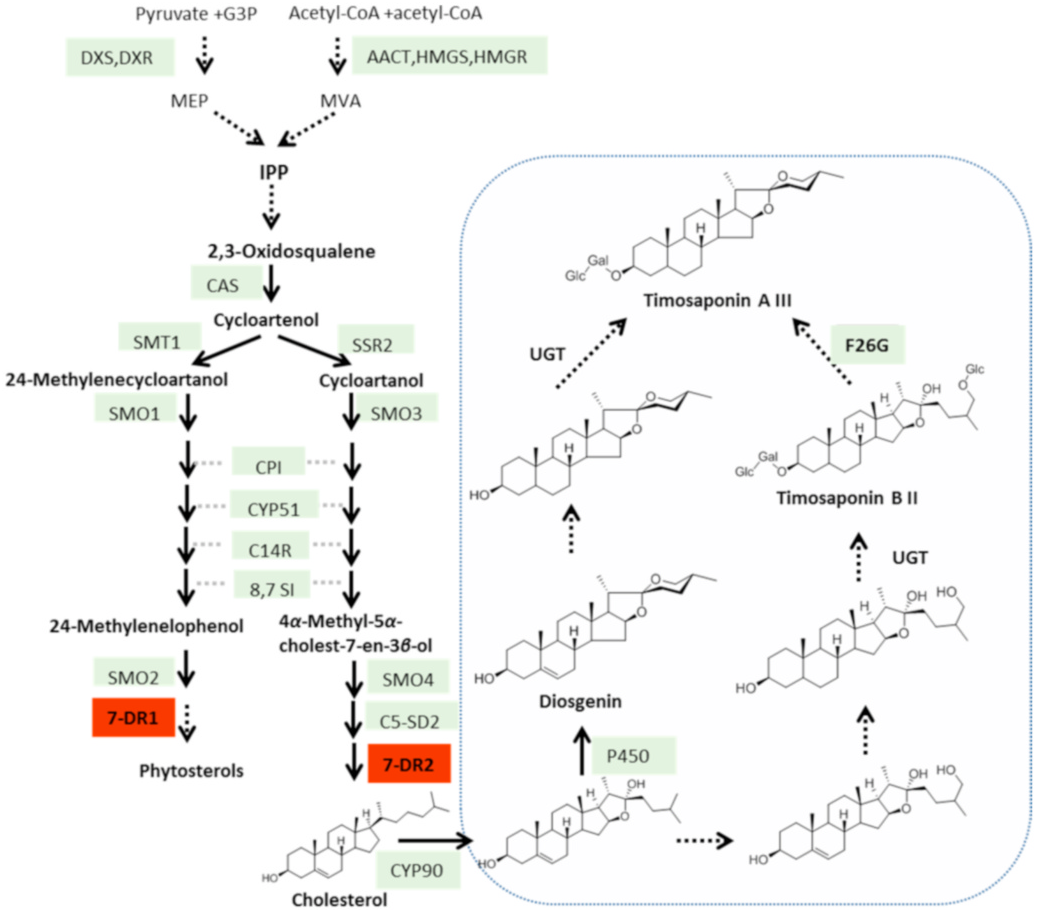
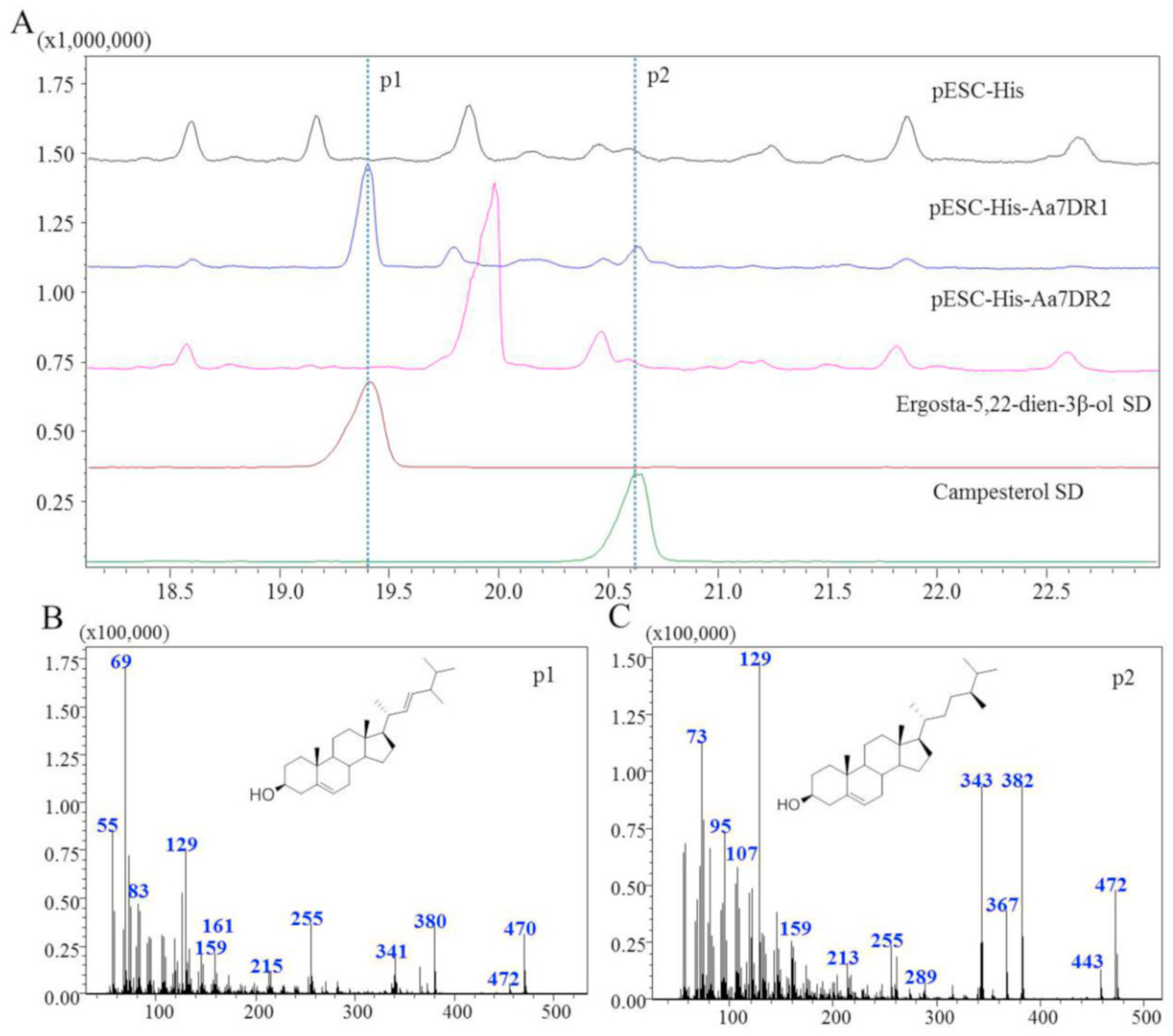
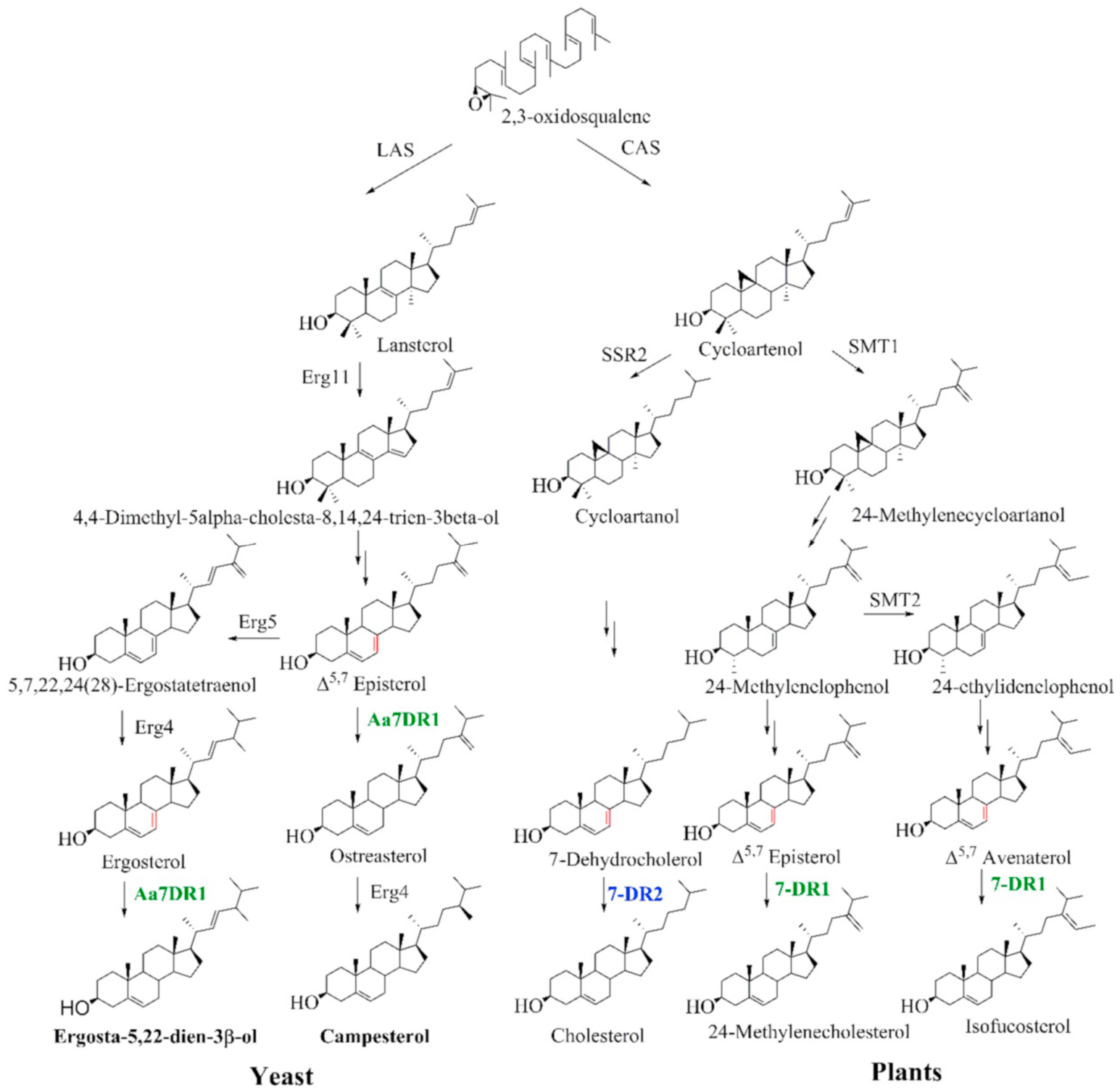
Yeast lacks the 7DR gene, and Δ 5,7 -eposterol can be used as a substrate for the heterologous 7DR enzyme ( Fig. 1 View Fig ). The two Aa7DRs were heterologously expressed in S. cerevisiae for functional characterization. Aa7DR1 and Aa7DR2 were cloned into the expression vectors pESC-HIS-Aa7DR1 and pESC-HIS-Aa7DR2, then were transformed into S. cerevisiae strain Cen.pk2-1D. Compared with the empty-vector-transferred control, the yeast strain expressing Aa7DR1 yielded two prominent products (peaks 1 and 2) at comparable levels ( Fig. 6A View Fig ). While there were no significant differences between pESC-His-Aa7DR2 and the control. To determine the structural features of Aa7DR1 products a large-scale fermentation (7 L) was used to accumulate enough products. The products were isolated and purified using open silica gel column and semi-preparative reversed-phase high performance liquid chromatography. The structures of p1 were confirmed as ergosta-5,22-dien-3β- ol by 1 H and 13 C-NMR data (Supplemental Figures S6 View Fig and S 7 View Fig ; Supplemental Table S6). Product p2 was identified as campesterol by 1 H-NMR and comparison with the standard. We determined Aa7DR1 possessed 7- dehydrocholesterol reductive function to reduce Δ 5,7 -eposterol and ergosterol at Δ 7 position. The indirect product campesterol (p2) was further catalyzed by endogenous gene erg4 on ostreasterol ( Fig. 5 View Fig ).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |