Bathymodiolus heretaunga
|
publication ID |
https://doi.org/10.5281/zenodo.197498 |
|
DOI |
https://doi.org/10.5281/zenodo.5662452 |
|
persistent identifier |
https://treatment.plazi.org/id/B43A87A0-BF5B-FA5D-6689-F8DDFB78FE5F |
|
treatment provided by |
Plazi |
|
scientific name |
Bathymodiolus heretaunga |
| status |
sp. nov. |
Bathymodiolus ( s. l.) heretaunga Saether, Little, Campbell, Marshall, Collins and Alfaro sp. nov.
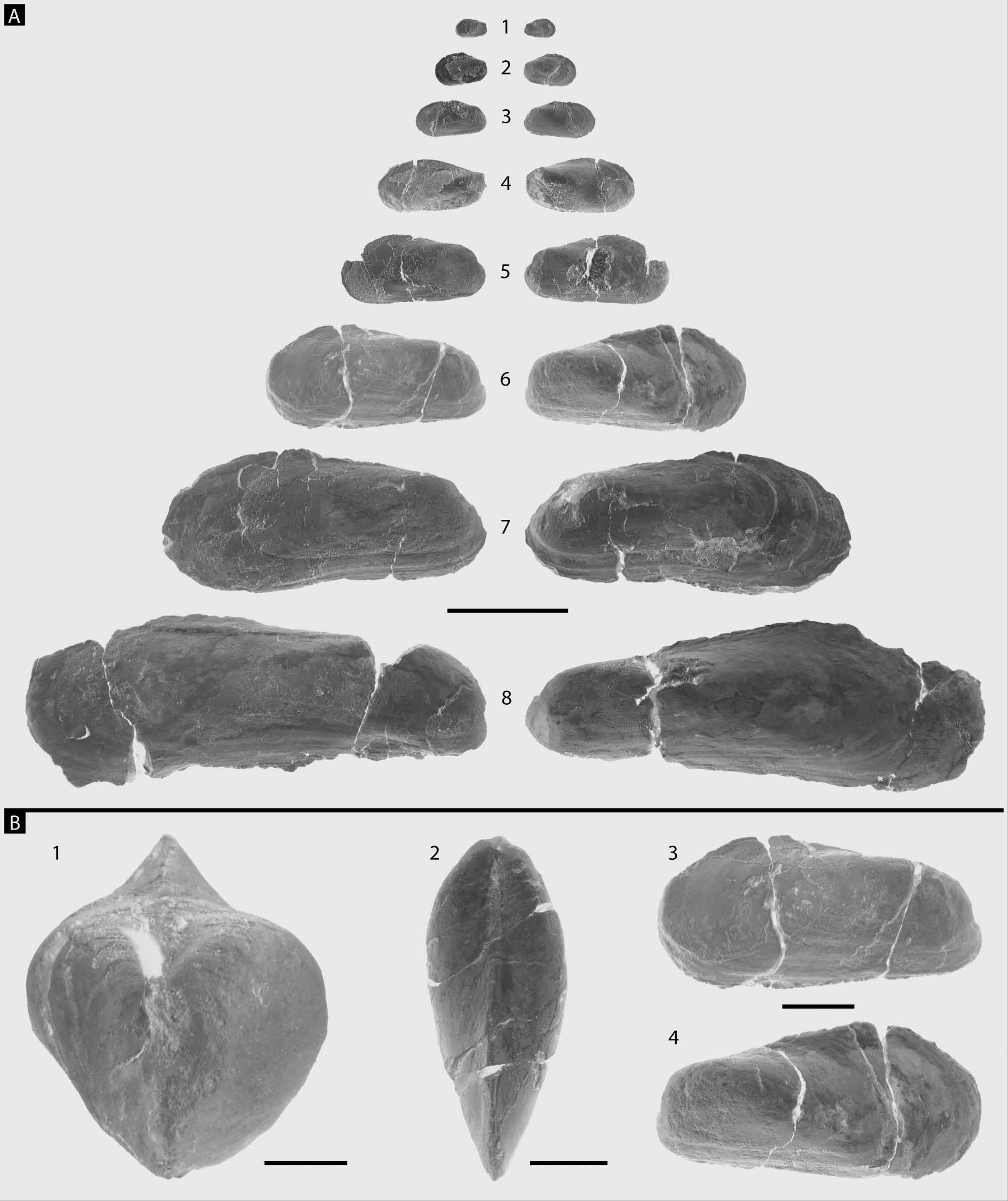
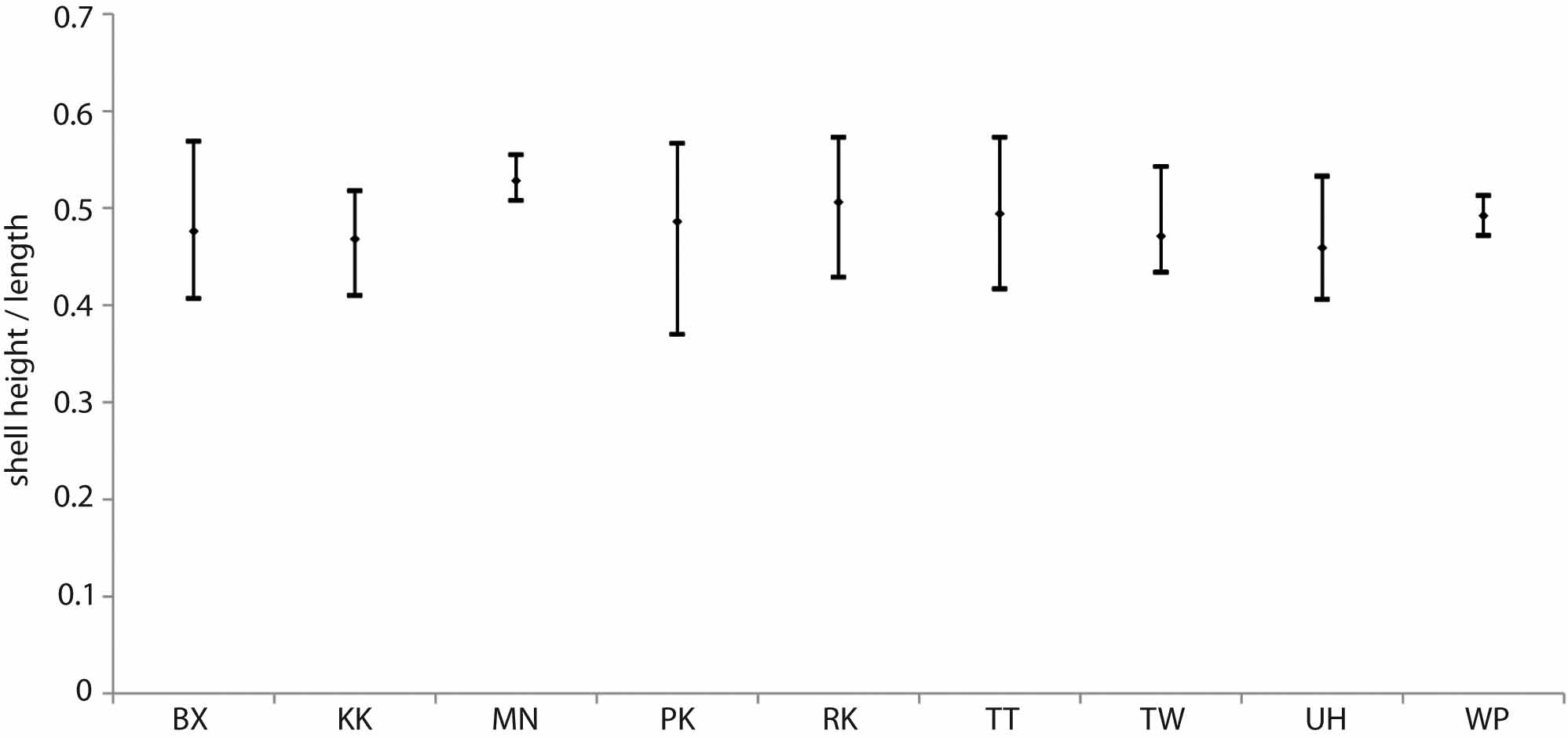
( Figs. 5 View FIGURE 5 , 6, 7 View FIGURE 7 , 10 View FIGURE 10 , 16 View FIGURE 16 )
?Mytilid closely resembling Idasola Beu & Maxwell, 1990 .
Bathymodiolus aduloides .— Collins, 1999: 33, pls. 1–4, Table 1 not Hashimoto & Okutani, 1994.? Bathymodiolus n. sp. A Collins, 1999.
Group 1 (G1) Collins, 1999.
Group 2 (G2) Collins, 1999.
Modiolus areolatus .— Collins, 1999: Table 1 not Gould, 1850. Xenostrobus cf. altijugatus Collins, 1999 not Marwick, 1931. Modiolus areolatus .— Campbell et al. 2008: 92, Table 1 not Gould, 1850. Xenostrobus cf. altijugatus Campbell et al. 2008 not Marwick, 1931. Smaller, stouter, flared variety that resembles ... Bathymodiolus aduloides Campbell et al., 2008 .
Holotype. Specimen TM8719, ( Figs. 5 View FIGURE 5 A.6, B.1–4), adult, UOA, borrowed from GNS.
Type locality. Puketawa (Y16/f0580), Hawke’s Bay, North Island, New Zealand;?late Early Miocene– Middle Miocene (Clifdenian–Lillburnian) hydrocarbon seep carbonate.
Paratypes. 17 moderately to well preserved small to large specimens. Two medium sized, L4171, L4178, from Karikarihuata (Y16/f1049, Y16/f1043); one medium sized, TM8717, one large, TM8724 ( Fig. 5 View FIGURE 5 A.8), from Puketawa (Y16/f0580); three small, L4099 ( Fig. 5 View FIGURE 5 A.1), L4100 ( Fig. 5 View FIGURE 5 A.2), L4201 ( Fig. 5 View FIGURE 5 A.3), four medium sized, L4071, L4084, L4166 ( Fig. 5 View FIGURE 5 A.5), L4198, one large, TM8727, from Rocky Knob (Y16/f0641, Y16/f1036, Y16/f1038, Y16/f1039, Y16/f1040, Y16/f1041, Y16/f1043); one small, TM8733, one large, TM8734 ( Fig. 5 View FIGURE 5 A.7), from Tauwhareparae (Y16/f0539); one small, L4066, two medium sized, L4068, L4088, from Totaranui (Y16/f1056). All specimens at UOA, five borrowed from GNS. See Table 4 for dimensions.
Other material. 199 poorly to well preserved small to large specimens: 19 from Bexhaven (Y16/f0566, Y16/f1032, Y16/f1048); nine from Karikarihuata (Y16/f0575, Y16/f1046, Y16/f1049, Y16/f1051, Y16/ f1052, Y16/f1053); two from Moonlight North (Y16/f1033, Y16/f1054); 10 from Puketawa (Y16/f0580); 88 from Rocky Knob (Y16/f0641, Y16/f0642, Y16/f1028, Y16/f1029, Y16/f1030, Y16/f1034, Y16/f1036, Y16/ f1037, Y16/f1038, Y16/f1039, Y16/f1040, Y16/f1041, Y16/f1042, Y16/f1043, Y16/f1044); nine from Tauwhareparae (Y16/f0539, Y16/f1055); 40 from Totaranui (Y16/f0575, Y16/f1056); 16 from Ugly Hill (U23/f0266); four from Wanstead (U23/f7464); two from Waipiro (Z16/f0075, Z16/f7491). See Appendix I for dimensions and specimen data. Approximately 97 others from all localities, too poorly preserved for verifiable identification and use in descriptions. All specimens at UOA, 53 borrowed from GNS.
Etymology. The Māori name for Hawke’s Bay, a region of New Zealand throughout which localities bearing fossils of this species are found; used as a noun in apposition.
Diagnosis. Shell rather small for group (L up to 95.4 mm), highly variable in shape; umbones situated at 5–14% shell length; umbonal region rather pronounced, angulated; anterior margin broadly rounded in some immature specimens; ventral margin slightly concave in some adult specimens, convex in immature specimens.
Description. Shell rather small for group (L up to 95.4 mm, H up to 35.0 mm, I up to 27.9 mm), elliptical in immature specimens, modioliform in adult specimens (some specimens cuneiform), essentially equivalve, rather thin (usually not preserved or expressed only in flaky patches), highly variable in shape but generally becoming slightly more elongate with growth, adult (L ≥ 38 mm) H/L = 0.37–0.57 (mean = 0.48), smaller specimens with H/L = 0.41–0.57 (mean = 0.50), of which small specimens (L < 15 mm) notably stouter with H/L = 0.44–0.57 (mean = 0.53). Umbones subterminal, prosogyrate, anterior, situated at 5–14% shell length, in most specimens 6–11%, placement consistent throughout growth; umbonal region rather pronounced and angulated, especially in smaller specimens, becoming more rounded and flattened with growth, covering ca. 15–20% of dorsal length in adult, less in juveniles. Anterior portion of shell short, slightly protrusive in some specimens. Anterior margin narrowly rounded, broadly rounded in some juvenile to half-grown specimens; dorsal margin strongly convex in smallest specimens, gradually becoming less convex with growth, more or less straight in largest specimens; posterodorsal corner usually weakly to strongly angular, broadly rounded in some specimens, marking point of greatest valve height at ca. 70–80% along shell length from anterior margin, only just in posterior in some early juvenile specimens; posterior margin broadly rounded, evenly in some specimens but typically sloping towards the ventral margin; ventral margin rather strongly convex in smallest specimens, becoming weakly convex to nearly straight in half-grown specimens, developing gentle concavity centred just in anterior in adult specimens or remaining more or less straight, in some specimens weakly to moderately convex in posterior third, especially in specimens in which concavity forms. Broadly rounded ridge runs from umbonal region, opening out to posteroventral and ventral margins, becoming obsolete before ventral margin, persisting to posteroventral margin in most specimens. External surface with irregular, fine, well developed commarginal growth lines and waves, rather strongly rugose medially in some specimens ( Fig. 6 A); growth waves and rugae typically rather strongly reflected on interior, especially in posterior and ventral portions. Hinge and ligament plate unknown. Ligament extending over ca. 85–90% of dorsal margin, running from beaks to in front of posterodorsal corner, terminating in moderately gentle taper. Subligamental ridge typically faint, strong in some specimens, running roughly parallel to ligament from near beak, becoming obsolete at 50–80% ligament length, visible from ventral but not lateral view. Muscle scars strongly impressed to faint, but generally poorly preserved; anterior adductor scar small, oval to elongate oval, located directly below beak close to anteroventral margin; posterior adductor scar medium to large, oval (long axis roughly parallel to posterodorsal margin), contiguous anterodorsally with posterior byssal retractor complex; anterior byssal retractor scar (known only from one specimen) small, oval, located in posterior of umbonal cavity, visible from ventral but not lateral view; posterior byssal retractors form long, thin, continuous scar complex, united with anterodorsal boundary of posterior adductor scar and running anteriorly (also slightly dorsally) in direction towards umbones, terminating at roughly middle of shell length; pallial line indistinct, running between posteroventral boundaries of anterior and posterior adductors, roughly parallel to ventral and posteroventral margins. Larval shell unknown.
Remarks. The new species is identified as a bathymodioline owing to its occurrence at hydrocarbon seep sites and the modioliform shell shape and size. It is assigned to the B. childressi clade mainly because of the scar of the posterior byssal retractors, which form a continuous scar united with the posterior adductor, reflecting the multibundle posterior byssal retractor complex diagnostic of modern species of this clade. This key feature distinguishes it from members of the B. aduloides and B. thermophilus clades, which have well separated anterior and posterior portions of the posterior byssal retractor. The new species can also be assigned to the B. childressi group (within the B. childressi clade) with some confidence. Each of the diagnostic characters of this group are satisfied in that the shell is small (range is small to rather large), the umbones are subterminal to almost terminal, and the anterior byssal retractor scar is located in the posterior part of the umbonal cavity. The only criterion that is not met precisely is the anterior margin, which is always narrowly rounded in the definition of the B. childressi group, but is broadly rounded in some immature specimens of B. heretaunga , although narrowly rounded in most specimens. There also are key features that exclude B. heretaunga from the other groups of the clade. The shells are too small and stout for the B. ( s. l.) edisonensis , B. ( s. l.) tangaroa and Gigantidas groups, and the position of the anterior byssal retractor scar is different from each. The new species cannot be placed in the B. ( s. l.) japonicus group, which consists solely of B. japonicus Hashimoto and Okutani, 1994 , because this species has the anterior byssal retractor scar located in the anterior portion of the umbonal cavity, and its anterior margin is not narrowly rounded in adult specimens. The subterminal umbonal placement of B. heretaunga eliminates inclusion within the B. ( s. l.) hirtus group, which contains species with umbones that protrude beyond the anterior margin of the shell. The new species is introduced as the smallest member of the B. childressi group, the largest specimen of B. heretaunga being 15% smaller than the next smallest described species, B. ( s. l.) mauritanicus Cosel, 2002 .
Bathymodiolus heretaunga has a quite distinct H/L ratio throughout ontogeny compared to any other member of the group, or for that matter the clade. Within the B. childressi group, the H/L ratio of the new species is closest to B. childressi Gustafson, Turner, Lutz and Vrijenhoek, 1998 but it is more variable, becoming more elongate when fully grown, and B. mauritanicus and B. ( s. l.) platifrons Hashimoto and Okutani, 1994 are yet stouter and less variable than B. heretaunga . Bathymodiolus heretaunga has a rather thin shell like B. childressi , but in B. mauritanicus and B. platifrons the shell is thick and solid. Immature specimens of the new species never develop concavity in the ventral margin unlike B. childressi , and ventral concavity is less marked in adult specimens than in B. mauritanicus . The umbones are situated more terminally in the other three species of the group than in the new species, which results in a more protrusive anterior portion of the shell in B. heretaunga . The umbones also are far more prominent and angulated in B. heretaunga than in any other species of the group. The ligament extends over a greater portion of the dorsal margin in B. heretaunga than in B. childressi , and ends in a moderate taper like B. childressi but unlike B. mauritanicus and B. platifrons , in which it ends abruptly. The subligamental ridge runs beneath the ligament for a similar length to B. mauritanicus , but is only visible from the ventral perspective, whereas it is visible from both ventral and lateral views in B. mauritanicus . The subligamental ridge also is only visible from the ventral view in B. platifrons , however it is typically more distinct in this species. The muscle scars of the new species are similar in size and location to the other species of the group, although they are always indistinct in B. childressi , but may be well impressed in B. heretaunga ( Fig. 6 D–F). The new species is most similar to the fossil Japanese species B. akanudaensis , with which it shares a similar lateral profile, but B. akanudaensis shows a lesser tendency towards elongation with growth, is generally more strongly inflated than B. heretaunga , and has slightly more terminal umbones. B. akanudaensis also is apparently less variable, and confirmed specimens of the Japanese fossil species have not been observed to reach as large a size as B. heretaunga . Furthermore, features of the internal shell of B. akanudaensis are unknown, and the muscle scars were a key factor in deciding the placement of B. heretaunga , therefore comparison on this important basis is impossible.
Although the majority of B. heretaunga specimens are small, occasionally anomalously large specimens have been collected, especially from Tauwhareparae and Totaranui (Table 4). These larger specimens are herein assigned to the same variable new species as the smaller ones on the basis of population-level variation, but it may be that larger specimens from these sites represent a different species. Speciation cannot be confirmed because details of features necessary for species-level identification are unavailable from the collected specimens. This size distribution pattern is also seen in collections of B. akanudaensis and unconfirmed larger mussel specimens from the same deposits in Central Japan, with the bulk of specimens of confirmed B. akanudaensis being relatively small but the larger mussel specimens sometimes reaching over 100 mm in length and being rather more elongate. Work on the taxonomic relationship among these differently sized specimens is ongoing (T. Nobuhara, pers. comm. 2009). Other intra-site variations also are recognized among the specimens of B. heretaunga of this study, again herein regarded as population-level differences in lieu of further specimen collection and establishment of important species-level identifying features. Some of the larger specimens from Bexhaven have more markedly concave ventral margins, and appear to develop one or two moderately strong ridges that run from the umbonal region posteriorly, but this is only observed in three specimens. Some specimens from the type locality Puketawa appear to have the umbones situated less anteriorly, with a more bean-shaped form. They more strongly resemble the shell shape of species of Adipicola Dautzenberg, 1927 , but there is not enough diagnostic detail in the specimens available for confirmation of this placement. Morphometric data of Puketawa specimens also reveal them to be the least divergent from the “typical” size and shape of B. heretaunga of any site population. Finally, specimens from Ugly Hill, the best-sampled of the southern hydrocarbon seep sites, generally appear to have a more cuneiform overall shape, formed by a more pronounced and narrowly rounded posterodorsal corner, although two Ugly Hill specimens provide muscle scar features that are consistent with others from the northern sites ( Fig. 6 H), and are more confidently regarded as conspecific.
Each of the sites that yielded specimens with suspected population variations also contained specimens that were morphologically close to others sites, suggesting an intra- and inter-site spectrum of shape and size that does not lend itself to separating species easily, especially because of the prevalently poor to moderate preservational condition of the specimens. Principal component analysis of shell height, length, and umbonal placement in those specimens that were preserved well enough for quantitative analysis also did not yield any obvious inter-site patterns (data not shown). The ranges and means of shell height/length ratios of specimens were compared between sites and shown to have considerable overlap, even where the sample data set was quite small and with more potential for anomaly ( Fig. 7 View FIGURE 7 ). The unsuccessful statistical and morphometric attempts to isolate clear variations in shell shape and size between and within sites support the decision to erect a single, variable species until such time as further evidence may prove otherwise.
| UOA |
UOA/HCPF University of Athens/Hellenic Collection of Pathogenic Fungi |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Bathymodiolus heretaunga
| Saether, Kristian P., Little, Crispin T. S., Campbell, Kathleen A., Marshall, Bruce A., Collins, Mike & Alfaro, Andrea C. 2010 |
Bathymodiolus aduloides
| Collins 1999: 33 |