Neolamprologus chitamwebwai, Verburg, Piet & Bills, Roger, 2007
|
publication ID |
https://doi.org/10.5281/zenodo.178953 |
|
DOI |
https://doi.org/10.5281/zenodo.5662479 |
|
persistent identifier |
https://treatment.plazi.org/id/B4043248-FF91-FFB5-26B6-28EAF2EFFBB6 |
|
treatment provided by |
Plazi |
|
scientific name |
Neolamprologus chitamwebwai |
| status |
sp. nov. |
Neolamprologus chitamwebwai View in CoL sp. nov.
( Figs. 2 View FIGURE 2 and 3 View FIGURE 3. A )
Type material. Holotype: SAIAB 56270, female, 56.5 mm SL, Cape Bangwe on the Bangwe peninsula near Kigoma, Tanzania ( 04°54'44" S, 29°35'55" E), 13/10/1997.
Paratypes: The material was collected from one locality on the Bangwe peninsula (Cape Bangwe, Fig. 4 View FIGURE 4 ), about midway in between both localities of N. walteri (Tembo Rock and Muzungu Beach) . SAIAB 58256 (7), 18.0– 60.6 mm SL, Cape Bangwe, near Kigoma, Tanzania ( 04°54'44" S, 29°35'55" E), 13/10/1997; SAIAB 57911 (10), 53.2–72.7 mm SL, Cape Bangwe, near Kigoma, Tanzania, 13/04/1998; SAIAB 57908 (1), 56.5 mm SL, Cape Bangwe, near Kigoma, Tanzania, 13/04/1998; MRAC 98-056-P-2 (1), 68.6 mm SL, Cape Bangwe, near Kigoma, Tanzania, 13/04/1998.
Topotypes: GMNH 4420 (14), Cape Bangwe, near Kigoma, Tanzania ( 04°54'44" S, 29°35'55" E), 8/7/ 1998.
Diagnosis. Neolamprologus chitamwebwai can be distinguished from N. crassus , N. falcicula , N. olivaceous , N. pulcher , N. splendens , N. helianthus and N. marunguensis by a smaller body depth (24.9–28.9 vs. 29.0–34.4 % SL, Table 1 View TABLE 1 and Fig. 5 View FIGURE 5 ) and by a smaller cheek depth (16.7–24.1 % vs. 24.3–29.0 % HL). Neolamprologus chitamwebwai differs from N. walteri in the linear relations between cheek depth and head length and between body depth and standard length (significantly different intercepts, P <0.002, Fig. 5 View FIGURE 5 ) with smaller cheek depth and smaller body depth for specific specimen size. Neolamprologus chitamwebwai further differs from N. walteri in habitat preference (large boulders with sand vs. rubble substrate with fine sediment), by having less pronounced markings on the dorsal and caudal fins ( Fig. 2 View FIGURE 2 ), by a larger premaxillary pedicel ( Figs. 2 View FIGURE 2 and 6 View FIGURE 6 ), and by larger maximum length (72.7 vs. 56.7 mm). Neolamprologus chitamwebwai differs from N. falcicula by having drab grey-brown juveniles (compared with bright yellow-orange fins of the juveniles of N. falcicula ; Konings, 1998), and perhaps by having more dorsal soft rays (8–10 vs. 7 for holotype of N. falcicula ). It differs from N. olivaceous , N. savoryi , N. splendens , N. pulcher , N. brichardi and N. helianthus by the absence of markings on the operculum. Neolamprologus chitamwebwai differs from all species in the complex except N. walteri and N. marunguensis (with uncertain status for N. falcicula ) by the number of upper and lower canines (8 and 6 vs. 6 and 4 respectively for all others, except 6 and 6 for N. helianthus ). With 33– 35 scales in the longitudinal series, N. chitamwebwai is distinguished from N. gracilis , N. splendens and N. helianthus (36–38 scales). With 6–9 gill rakers, N. chitamwebwai is distinguished from N. brichardi and N. gracilis (10–18 gill rakers). Neolamprologus chitamwebwai differs from N. splendens , N. marunguensis and N. savoryi by the absence of scales on the paired fins and by the absence of ctenoid scales on the dorsal and anal fins; from N. savoryi , N. brichardi , N. olivaceous and N. pulcher by the presence of cephalic pits; from N. olivaceous and N. pulcher by the absence of conspicuous spots on scales and from N. brichardi , N. pulcher , N. helianthus , N. crassus , N. splendens and N. marunguensis by a smaller caudal peduncle depth (11.2–12.8 % vs. 13.4–15.3 % SL). Neolamprologus chitamwebwai differs from N. savoryi and N. gracilis by having less scales between the upper and lower lateral lines (2 vs. 3) and by having less scales around the caudal peduncle (16 vs. 19). Neolamprologus chitamwebwai further differs from N. savoryi by the absence of bars on the body, by a smaller predorsal fin length (29.4–33.3 % vs. 34.6–36.8 % SL) and by having more pectoral fin rays (13 vs. 12).
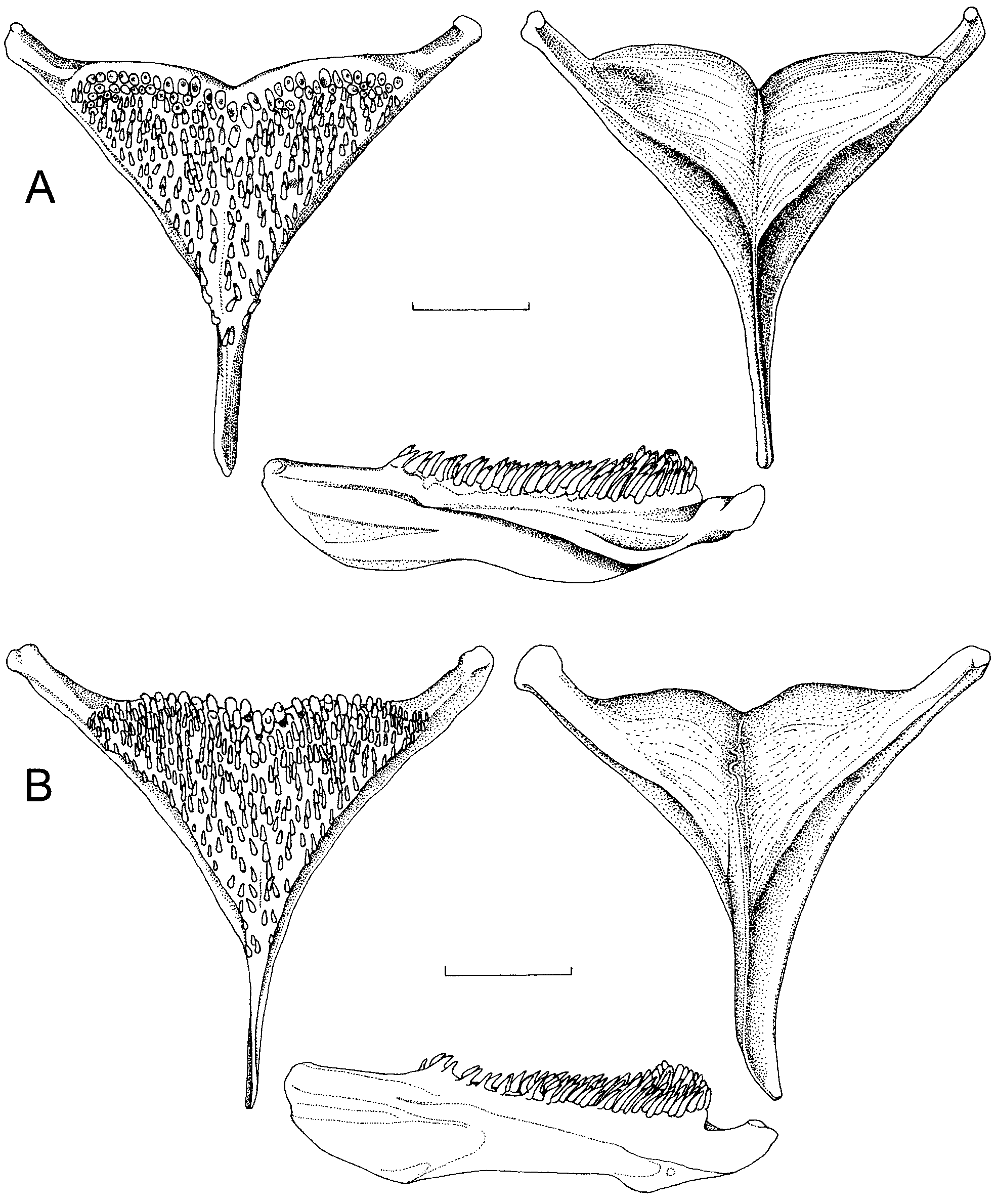
Description. Counts and measurements for the type series are given in Table 1 View TABLE 1 . Range of standard length of investigated specimens: males 53.2–72.7 mm ( 8 specimens), females 34.8–60.6 mm ( 6 specimens). A relatively elongate species (BD 24.9–28.9 % SL, mean 27.1 % SL). Head profile in front of eye convex. Premaxillary pedicel pronounced ( Figs. 2 View FIGURE 2 and 6 View FIGURE 6 ). Mouth terminal, isognath, gape ends well anterior to eye.
No sexual dimorphism, apart from what may be caused by a combination of allometry and the difference in sizes of males and females. For instance, pectoral fin length is larger in males, due to positive allometry.
Six-9 canines in the upper jaw (mode 8, 79 %) and 4–6 lower canines (mode 6, 86 % of specimens), with two outer canines on both jaws enlarged ( Fig. 6 View FIGURE 6 ). Dentigerous arm of premaxilla slightly recurved.
Gill rakers 6–9 ( Fig. 6 View FIGURE 6 ), slender, with black spots, decreasing in size towards anterior end of arch, often the first one or two bifid. Changes from posterior to anterior end of arch from flattened to rounded rakers.
No scales on cheek, in some specimens cycloid scales on preoperculum (not seen on the other species in the complex), few scales posterior of eye, above preoperculum, 10 – 18 cycloid scales on operculum, few cycloid scales in supra-occipital region in most specimens (posterior to eye), fully scaled cycloid on nape, about 5 –10 scales between pelvic and pectoral fin, urogenital area ctenoid scales, flank scales ctenoid. Few scales on dorsal and anal fin. No scales on pectoral and pelvic fins. Caudal fin small ctenoid scales, on 70–90 % of tail along middle rays in adults, range of scales on caudal fin increases with size (juveniles 25–35 mm: 25–30 % of tail scaled). Scales on outer rays range in adults up to past posterior end of middle rays. On juveniles no scales on fins, except tail, with less scales on tail than adults.
In the longitudinal line 33–35 scales, and relatively high number of scales in upper lateral line, 23–30. In upper lateral line 21–28 canal scales (76–100 % of all scales in the upper lateral line, median 25 canal scales, 94% of all scales). In lower lateral line 4–13 canal scales (50–100% of all scales in the lower lateral line, median 9, 77 %). Scales without canal segments in between canal scales were pitted. Most scales anterior to canal scales in lower lateral line were pitted. In few specimens 1 canal scale beyond the caudal flexure. In 6 specimens (43 %) upper lateral line continued for 1–3 scales on the caudal peduncle, 1 scale row further down than row containing upper lateral line on the flank, leaving on the peduncle only 1 scale row between upper and lower lateral lines instead of 2 scale rows ( Fig. 10 View FIGURE 10 ). No relation with size apparent.
First pelvic fin ray longest. Caudal fin lunate. Anal fin filament on average 4.2 % longer than dorsal fin filament. Dorsal fin margin concave ( Fig. 2 View FIGURE 2 B).
Lower pharyngeal jaw about as wide as long ( Fig. 7 View FIGURE 7 ). Dentigerous area covered with slender, pointed, unicuspid teeth, anteriorly slightly recurved, posterior rows straight and enlarged. On upper pharyngeal jaw teeth bicuspid with major cusp protracted.
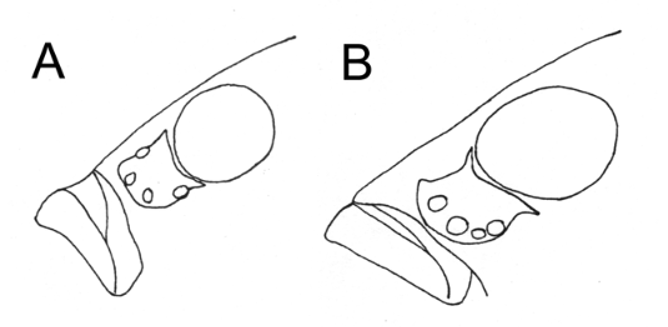
Post-lachrymal infraorbital bone series absent ( Fig. 8 View FIGURE 8 ). Narrow suborbital groove, consisting of almost separate cephalic pits, with superficial neuromasts. Papillae in pits not always visible.
Hardly any epidermal papillae present on body.
Coloration. Lighter coloured than N. walteri , grey to beige. Black margin on dorsal fin absent or only very thin, shorter and less conspicuous than in N. walteri . Yellow-brown vertical stripes, between the rays, on all fins, except the pectoral. On the dorsal and anal fin the stripe pattern is diagonal to the direction of the rays, about 4 stripes cross each ray. On the caudal fin stripes perpendicular to the rays (± 6 stripes). On pelvic fin stripe pattern is more or less perpendicular to rays. In preserved specimens colour of fins not or less discernible, banded patterns visible. The yellow-brown vertical stripes on the fins hardly noticeable when observing the fish in their habitat. The pectoral fin yellow at distal end. Iris light iridescent blue. Blue suborbital stripe, as found in many lamprologines, in location of sensory groove. Posterior margin of scales brownish.
Juveniles. Brown, smallest examined specimen almost yellow ( 16.9 mm SL), no bands on body, tail emarginate, no filaments on tail or anal and dorsal fins. Pelvic fins blackish, dorsal and anal fin dark to a lesser degree, no vertical stripes on fins. Margin of dorsal fin black. Little spots on the head. No scales on fins, except tail. On tail fewer scales than adults. Operculum and nape scaled, no scales on cheek and occiput.
Distribution. We found it only at one site on the Bangwe peninsula. However, Konings (1998) contains a photo of a specimen which may be N. chitamwebwai (pg. 80, ascribed to N. falcicula ), from the east coast near the centre of the lake (Lumbye). Bangwe peninsula has been an island for at least a part of the 19th century, when lake levels were about 10 m higher than at present ( Stanley, 1895, Nicholson, 1999).
Habitat and biology. Fig. 9 View FIGURE 9 shows N. chitamwebwai in its habitat. The habitat of N. chitamwebwai is strikingly different from that of N. walteri . It consists of large boulders, with a diameter in the order of 1 to 2 meter, on a steep slope, with some sand in between the stones, and no bivalve shell accumulations or other rubble present. The habitat of N. chitamwebwai is more exposed to currents, compared with that of N. walteri , with lower sedimentation rates, coarser sediment, and higher visibility. Such habitats are generally found at capes along the coast. For instance at the Bangwe peninsula, N. chitamwebwai is found at a site on the lake side of the peninsula, while N. walteri occurs on the more sheltered north and south shores of the peninsula.
The population density of N. chitamwebwai is much lower than that of N. walteri . Neolamprologus brichardi and N. savoryi occur often in close proximity with N. chitamwebwai . Neolamprologus chitamwebwai dives for cover when approached. The presence of sand in the habitat is mandatory. Neolamprologus chitamwebwai creates tiny refuges by removing sand at the foot of large stones. In areas without sand there are generally not enough spaces for refuge nor the opportunity to create them through digging. It spends the night in these crevices. Depth range 10– 30 m. Most common at 10– 20 m. Large depth overlap with N. brichardi , but N. brichardi reaches higher densities in shallower depths. Neolamprologus chitamwebwai has strong site fidelity, with only a small home range, about 2 m diameter. Observed to ingest sediment and spit out through mouth. An inspected oesophagus was filled with sediment. Young of < 10 mm were not observed.
Etymology. The species is named after Deonatus Chitamwebwa, the interim director of the Tanzanian Fisheries Research Institute during the period that the collections were made, in gratitude for his excellent hospitality and assistance and because of his elongated shape and small body depth to length ratio that makes him stand out as it does with N. chitamwebwai .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |