Oligoryzomys utiaritensis J. A. Allen, 1916
|
publication ID |
https://doi.org/10.5281/zenodo.210257 |
|
DOI |
https://doi.org/10.5281/zenodo.5662714 |
|
persistent identifier |
https://treatment.plazi.org/id/B1422D55-FFE8-FFF0-FF43-FADE207CF9F0 |
|
treatment provided by |
Plazi |
|
scientific name |
Oligoryzomys utiaritensis J. A. Allen, 1916 |
| status |
|
Oligoryzomys utiaritensis J. A. Allen, 1916
( Figures 2 View FIGURE 2 , 3 View FIGURE 3 , 4 View FIGURE 4 )
Holotype. AMNH 37541, adult female ( Figures 2 View FIGURE 2 and 3 View FIGURE 3 ), collected by Leo E. Miller on January 30th, 1914; measurements of holotype are provided in Table 2.
Type Locality. Brazil, Mato Grosso state, Rio Papagaio, Sapezal municipality, Utiariti; geographical coordinates 12°59’07’’S, 55°36’23’’W (taken by GPS).
Geographic Distribution. The six known collecting localities of O. utiaritensis ( Figure 1 View FIGURE 1 , Appendix 1) are distributed across the northwest of Mato Grosso and southwest of Pará. The area includes the Chapada dos Parecis, a massive plateau in northwestern Mato Grosso that marks the transition between the Cerrado and Amazonian domains in Central Brazil. All localities are restricted to the Rio Tapajós and Rio Xingu watershed. Carvalho (1960) did not specify where the specimens collected at Gradaús (Pará state) and identified by him as O. utiaritensis were housed – thus their identity was not confirmed; nevertheless, this locality, nowadays at the municipality of São Félix do Xingu ( 06°38' S, 51°59' W), is near the known range of the species and therefore could represent a seventh locality for O. utiaritensis .
External and cranial Measurements. See Table 2.
Diagnosis. A medium-sized Oligoryzomys species characterized by: (1) grizzled yellowish-brown dorsal pelage, contrasting with the whitish ventral pelage and tail weakly bicolored; (2) long incisive foramina, the posterior borders reaching or almost reaching the alveolus of the first upper molars, but never extending posteriorly; (3) the highest diploid number (2n=72) among Oligoryzomys species; and (4) three putative synapomorphies in cytochrome b, and one in intron 7 of beta fibrinogen (alignments available from the authors).
External Characters. Adult dorsal pelage grizzled yellowish-brown, between Antique Brown and Dresden Brown ( Ridgway, 1912), composed of long guard hairs and slightly shorter overhairs with a sub-apical brown-yellowish band. Lateral color lighter than in dorsum and with a clearly defined limit with the whitish ventral pelage. Ventral hairs white at their upper half and gray at their basal half. Internal surface of pinnae with brown-yellowish hairs; external surface brownish. Dorsal surface of feet covered with withish hairs, sometimes washed with cream color; short tufts of white ungual hairs at bases of claws on dII–dV. Tail longer than combined length of head and body, sparsely haired, and covered with more or less conspicuous epidermal scales, lacking a long tuft of terminal hairs and weakly bicolored, dorsal surface dark gray and ventral surface light gray (some specimens are unicolored in the distal half of the tail). Superciliary, genal, and mystacial vibrissae not extend beyond ears. Juveniles with grayish dorsum, with whitish ventral surface. Presence of eight mammae in inguinal, abdominal, postaxial, and pectoral positions.
Cranial Characters. Delicate skull, medium and narrow rostrum, but slightly wider than interorbital region; interorbital region hourglass-shaped. Braincase without supraorbital and postorbital ridges and with weakly developed lambdoidal ridge. Interparietal bone as broad as parietal. Relatively large zygomatic plate with deep zygomatic notch. Jugal bone absent, resulting in zygomatic process of squamosal in contact with the zygomatic process of maxillary. Incisive foramina with almost parallel margins, the posterior borders reaching or almost reaching the alveolus of the first upper molars, but never extending posteriorly. Palate with single large posterolateral palatal pits not recessed in palatine fossa. Palatal bridge broad and long. Bony roof of mesopterygoid fossa perforated by large sphenopalatine vacuities. Width of parapterygoid plate slightly greater than width of mesopterygoid fossa. Alisphenoid strut absent (buccinator-masticatory foramen and accessory foramen ovale confluent), alisphenoid canal with large anterior opening. Stapedial foramen and the posterior opening of the alisphenoid canal large, but squamosal–alisphenoid groove and sphenofrontal foramen absent (= carotid circulatory pattern 2; Voss 1988). Posterior suspensory process of the squamosal absent. Large subsquamosal fenestra, slightly smaller than postglenoid foramen. Periotic exposed posteromedially between ectotympanic and basioccipital, extending anteriorly to carotid canal. Mastoid perforated by conspicuous posterodorsal fenestra. In mandible, capsular process of lower incisor alveolus well developed in most adults; superior and inferior masseteric ridges converging anteriorly as open chevron below m1.
Dental Characters. Upper and lower incisors opisthodont; molars pentalophodont. Superior molar rows parallel. The first upper molar (M1) anterocone is divided into anterolabial and anterolingual conules by a weak anteromedian flexus only in young animals; animals with moderate wear lack anteromedian flexus; the anteroloph is well developed, separated from anterocone by a persistent anteroflexus; mesolophs are present on all upper molars in most specimens, but absent in specimens with moderate or heavy wear; the paracone is connected by an enamel bridge to the anterior moiety of protocone, and in some specimens to the mesoloph by the paralophule. M2 morphology is similar to M1 except in the anterior region: M1 has an anterocone, while M2 only has an anteroloph. Third upper molar (M3) is reduced, and has a single posterior cup, which we equate to the hypocone; hypoflexus is diminutive. Anteroconid of first lower molar (m1) without anteromedian flexid; the anterolabial cingulum is present on all lower molars; the anterolophid is present on m1 but absent on m2 and m3; an ectolophid is absent on m1 and m2; the mesolophid is distinct on unworn m1 and m2, sometimes joined to the entoconid; the posteroflexid is present on m3.
Karyotype. Karyotypic analyses of 25 specimens of Oligoryzomys utiaritensis showed 2n=72, FNa=76 (Figure 4). Autosome complement comprising three pairs of small-sized biarmed chromosomes and 32 acrocentric pairs, three large-sized and 29 varying in size from medium to small. X chromosome large submetacentric and small Y chromosome.
Habitat. Specimens of Oligoryzomys utiaritensis were recently captured between 110 and 570 m of altitude, in secondary semideciduous forest in the limits of corn and soy plantations, in eucalypt plantations with herbaceous vegetation, and in very altered vegetation (semi-deciduous forest) near plantations. No information about the vegetation of the type locality was provided in the description of O. utiaritensis ( Allen, 1916) , but the vegetation along the Papagaio River, including in the Utiariti region, is gallery forest.
Comparisons. Oligoryzomys utiaritensis differs from all other Oligoryzomys species by its unique karyotype. In addition, O. utiaritensis differs from other Oligoryzomys that occur in Brazil by a combination of other characters including (1) whitish ventral pelage, (2) a sharply defined limit between lateral and ventral pelage color, in comparison to a buffy venter without defined limit between lateral and ventral coloration in adult specimens of O. moojeni , O. fornesi and O. flavescens (see table 1); (3) slightly bicolored tail, contrary to unicolored tail in O. nigripes and O. rupestris (other species of the genus also have slightly bicolored tails); (4) a large size (adult HBL> 91 mm in average) comparable to O. nigripes , O. stramineus , and O. chacoensis , and opposed to small size species O. rupestris , O. microtis , O. moojeni , O. flavescens , O. delicatus , O. messorius , O. fornesi (HBL < 90 in average).
In addition, we present here more detailed comparison between O. utiaritensis and O. moojeni in view of their closer geographic distribution and their molecular and karyotypic similarities. In Oligoryzomys utiaritensis , the dorsum is yellowish-brown (unlike the reddish-brown dorsum of O. moojeni ), with a clearly defined limit between dorso-lateral and ventral pelage (unlike the poorly defined limit in O. moojeni , forming a gradient between lateral and ventral coloration). In O. utiaritensis , the rostrum is slightly wider than the interorbital constriction (unlike O. moojeni in which the rostrum and interorbital constriction are of similar width), and the subsquamosal fenestra is almost of the same size of postglenoid foramen (unlike O. moojeni with a smaller subsquamosal fenestra about half the size of the postglenoid foramen).
Etymology. Oligoryzomys utiaritensis was named by J.A. Allen based on the type locality Utiariti (spelled Utiarity in old maps and accounts), referring to a Amerindian village in Sapezal municipality.
Specimens examined. See Appendix 1.
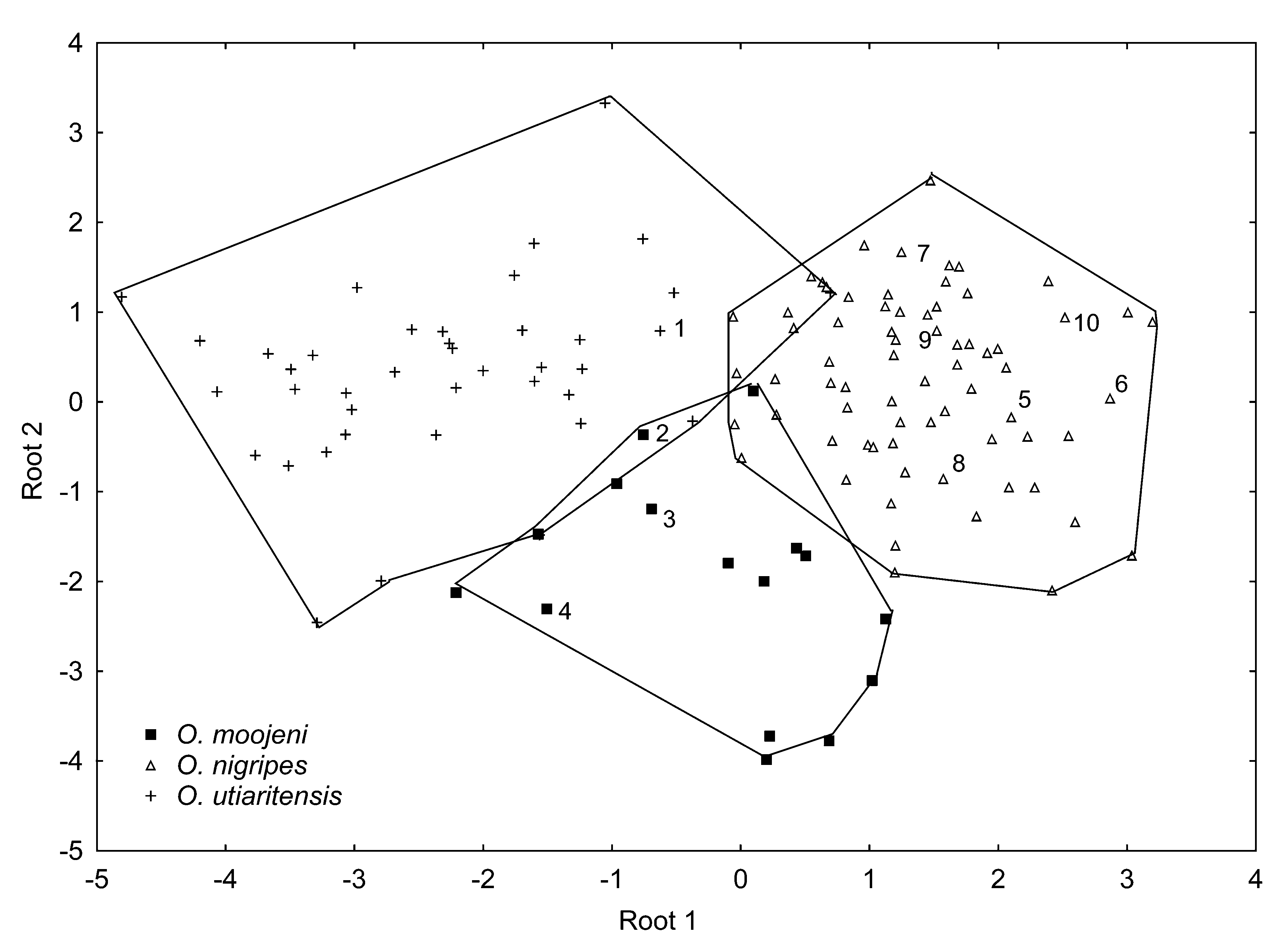
Remarks. The specimens referred as O. utiaritensis from Conceição do Mato Dentro ( Avila-Pires, 1960) and Caratinga ( Botelho & Williams, 1980; Botelho et al., 1981; Linardi et al., 1984) are herein identified as O. nigripes , based on morphometric ( Figure 4 View FIGURE 4 ) and morphological analyses of the voucher material deposited at the Museu Nacional (see voucher numbers below in Appendix 1). Myers and Carleton (1981) discussed the composite nature of the paratype of O. utiaritensis (AMNH 37540); our examination of this specimen was in agreement with their assessment: skull and skin do not belong to the same animal, the skin being from a larger animal than the skull. We thus remove this specimen from the type series of O. utiaritensis .
Oligoryzomys phylogenetics. Our phylogenetic results were generally coincident with recent studies of Oligoryzomys (Francés & D'Elía, 2006; González-Ittig et al., 2010; Miranda et al., 2009; Palma et al., 2005, 2010; Richter et al., 2010; Rivera et al., 2007; Rogers et al., 2009). Analyses of an additional marker, the nuclear i7 FGB gene, provided independent corroboration, usually with high nodal support (especially for the Bayesian analysis), of several clades found with mitochondrial cyt- b.
We could observe that O. utiaritensis was found in one the major lineages recovered by the cyt- b analyses with 6 other species: (( O. messorius , (( O. destructor , O. rupestris ), ( O. delicatus , (Oligozyzomys sp., ( O. moojeni , O. utiaritensis )))); in the i7 FGB analyses, O. rupestris , O. moojeni , and O. utiaritensis also clustered together (the other species were not available for these analyses).
Our phylogenetic reconstructions were also the first to include O. rupestris , which appeared as sister group to O. destructor with respect to the ( O. moojeni , O. utiaritensis ) clade in the cyt- b phylogeny and as a sister lineage of O. moojeni in the i7 FGB phylogeny. Nevertheless, our results confirmed the need for analyzing additional loci to reach a more complete understanding of Oligoryzomys phylogeny, mainly because analyses exclusively based on cyt- b did not provide sufficient phylogenetic signal for a robust resolution of several interspecific relationships.
| AMNH |
American Museum of Natural History |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.