Dasyhelea mesophylla Dominiak and Borkent, 2023
|
publication ID |
https://doi.org/ 10.1080/00222933.2023.2203336 |
|
DOI |
https://doi.org/10.5281/zenodo.8070230 |
|
persistent identifier |
https://treatment.plazi.org/id/AC0787A9-FFC5-0A46-FF34-C9EBFC7FFCC4 |
|
treatment provided by |
Plazi |
|
scientific name |
Dasyhelea mesophylla Dominiak and Borkent |
| status |
sp. nov. |
Dasyhelea mesophylla Dominiak and Borkent , sp. nov.
Diagnosis
The only Dasyhelea in the Neotropical Region with the following characteristics. Male adult: with a single elongate radial cell, sternite 9 straight, paramere sinusoidal, twisted and tapering to a narrow apex, fused basally with gonocoxal apodemes and forming with them an asymmetrical structure, aedeagus symmetrical with dark, well-developed anterolateral club-like projections and 2 well separated and slender posterior projections with apices that are hooked dorsolaterally. Female adult: with 1 elongate radial cell, frontal sclerite broader than long, with sternite 9 elongate anteriorly, conical and with a rounded apex. Pupa: with the respiratory organ curved and coming to a sharp point, with a double row of about 7 circular pores each (so about 14 total) restricted to the apical 0.3 of the respiratory organ, and with a slightly elongate, pointed, gradually tapering terminal process bearing 2–3 pointed tubercles laterally near its base. Larva: not presently diagnosable, but see taxonomic discussion.
Description
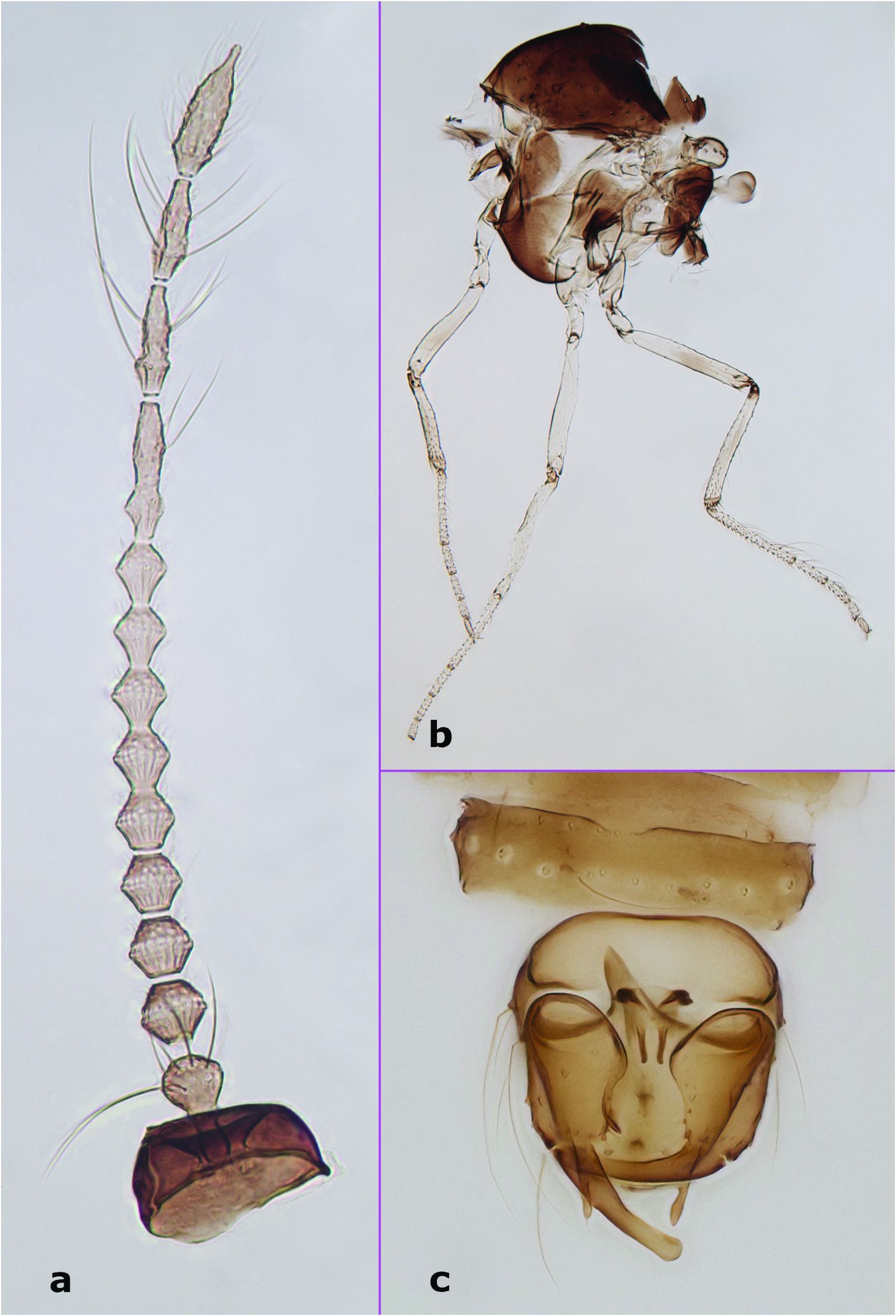
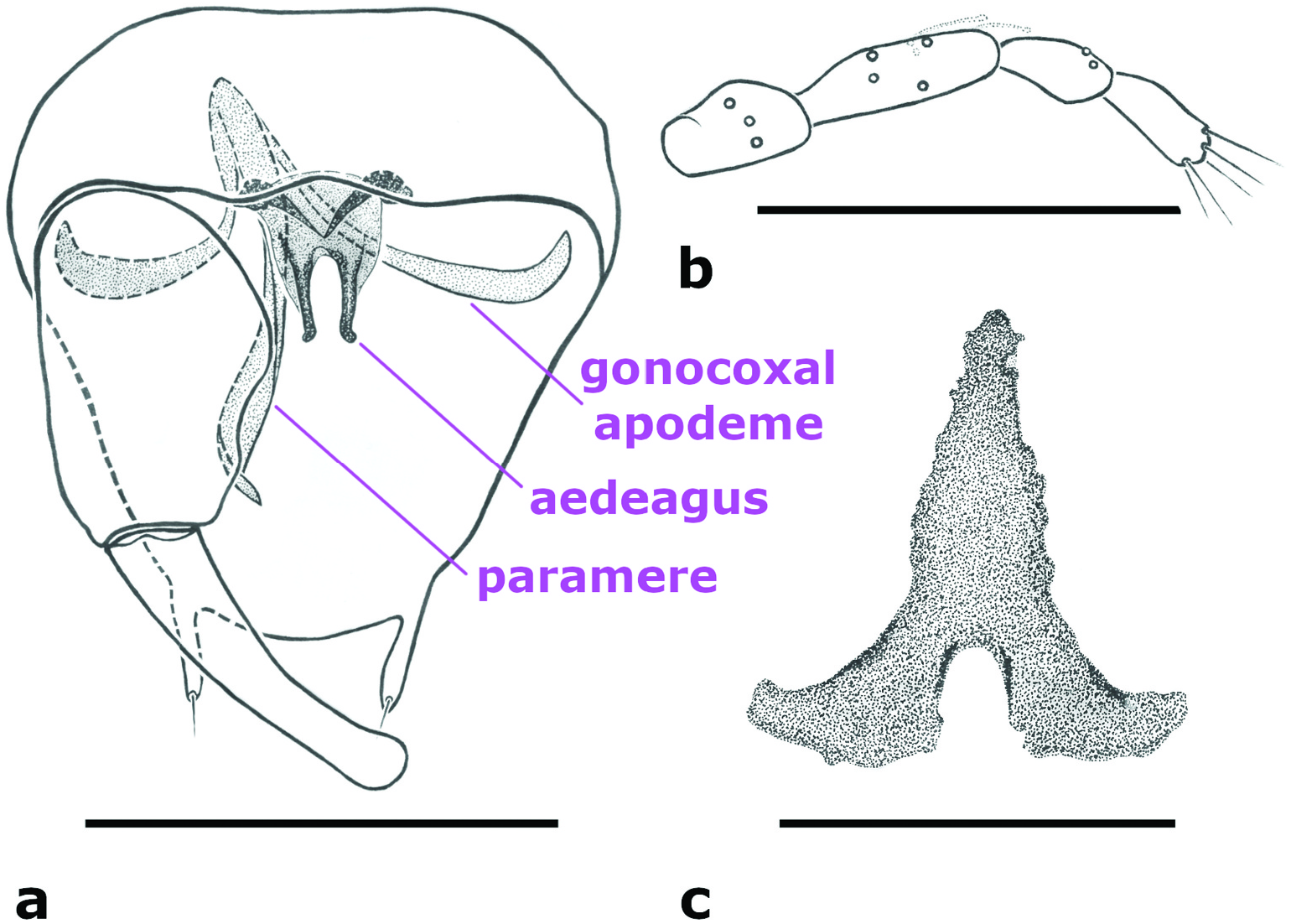
Male adult. Antenna ( Figure 1 View Figure 1 (a)) dark; antennal flagellum length 0.58–0.60 mm (n = 3), antennal ratio 0.79–0.95 (n = 3). Frontal sclerite broad, with long, slender projection. Clypeus divided into two parts, with 7–11 setae distributed laterally in two rows (n = 4). Palpus ( Figure 5 View Figure 5 (b)) with third palpal segment relatively stout, 44–53 μm long (n = 4); palpal ratio of the third segment 2.54–3.17 (n = 4); sensilla capitata present on segment 3 only, rather sparse and distributed closer to its outer margin. Thorax ( Figure 1 View Figure 1 (b)), aside from slightly lighter lateral sclerites, dark. Scutellum yellowish, with 6–8 bristles and 1–5 smaller setae (n = 5). Wing with 1 radial cell; wing length 0.76–0.81 mm (n = 4), costal ratio 0.45–0.48 (n = 4). Halter dark. Legs ( Figure 1 View Figure 1 (b)) pale with indistinct, irregular darker patches at midlength of fore- and midfemora and tibiae of all legs, and apical half of hind femur. Hind tibial comb with 5–7 spine-like setae (n = 4). Tarsal ratios: foreleg 2.1–2.3 (n = 3), midleg 2.4–2.6 (n = 3), hind leg 2.2–2.5 (n = 4). Abdominal segments 3–8 well sclerotised; sclerotisation on segments 3–5 disjunct medially. Genitalia ( Figures 1 View Figure 1 (c), 5(a)). Apicolateral process of tergite 9 prominent, finger-like, with single apical seta. Cercus moderately small, with 4 setae (n = 1). Posterior margin of sternite 9 straight or with shallow excavation medially. Gonocoxite without mesoventral hook. Gonostylus nearly straight, with rounded apex, same length as or slightly longer than gonocoxite. Paramere and gonocoxal apodemes fused and forming an asymmetrical structure; paramere sinusoidal, twisted, tapering to narrow apex. Aedeagus symmetrical, with dark, well-developed anterolateral club-like projections, 2 well-separated and slender posterior projections with apices hooked dorsolaterally; anteroventral margin of aedeagus weakly sclerotised, rounded.
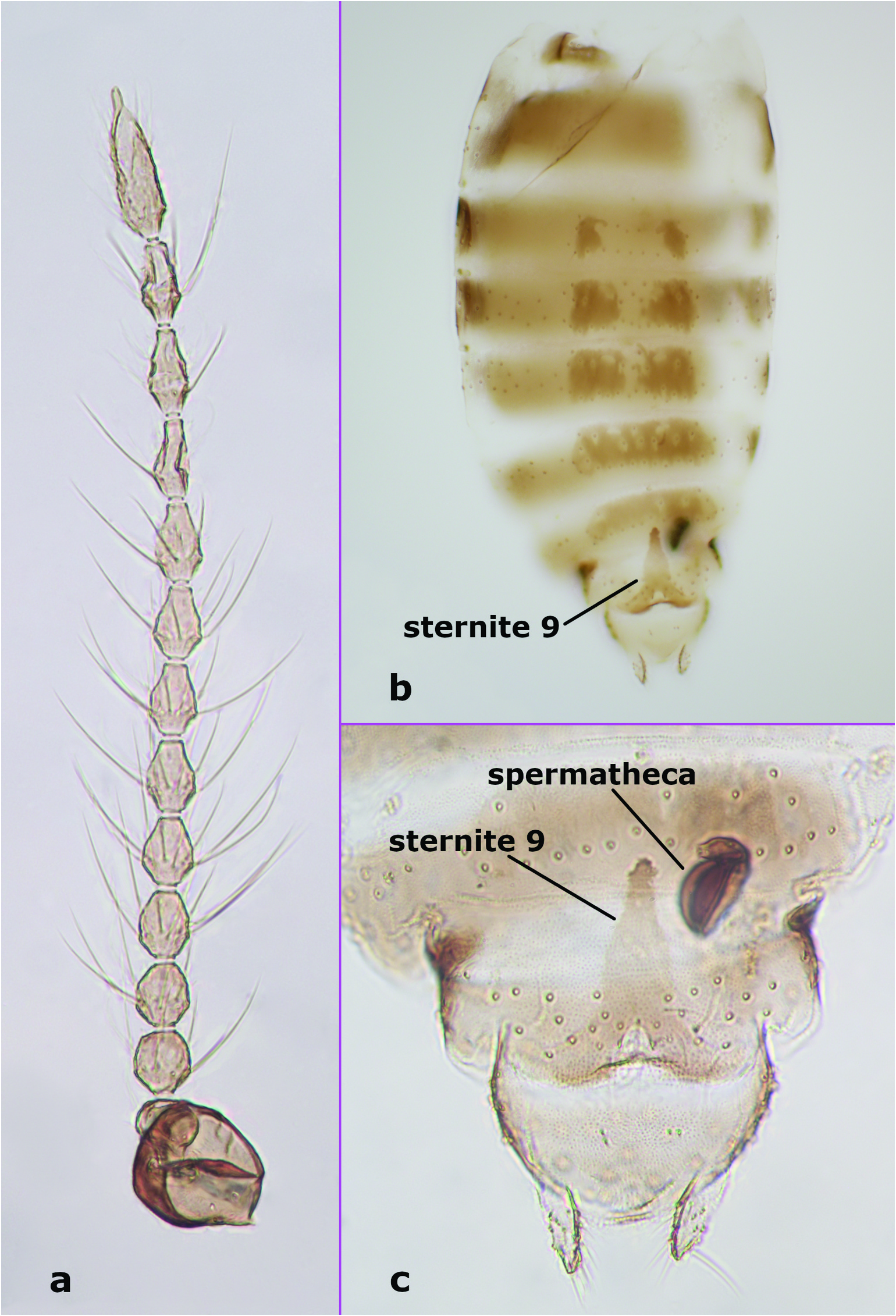
Female adult. Antenna ( Figure 2 View Figure 2 (a)) dark; antennal flagellum length 0.52 mm, antennal ratio 0.84. Frontal sclerite broad, with long, slender projection. Clypeus divided into two parts, with about 15 setae distributed laterally in two rows. Third palpal segment relatively stout, 46 μm long; palpal ratio of the third segment 2.67; sensilla capitata as in male. Scutellum yellowish, with 8 bristles and about 8 smaller setae. Wing with 1 radial cell; wing length 0.78 mm, costal ratio 0.50. Legs pale with indistinct, irregular darker patches at midlength of fore-, hind femora, tibiae. Hind tibial comb with 6 spine-like setae. Tarsal ratios: foreleg 2.3, midleg 2.3, hind leg 2.3. Genitalia ( Figures 2 View Figure 2 (b,c), 5(c)). Abdominal segments ( Figure 2 View Figure 2 (b)) 3–8 well sclerotised; sclerotisation on segments 3–5 disjunct in the middle. Sternite 9 elongate anteriorly, conical, with rounded apex ( Figures 2 View Figure 2 (b), 5(c)). Spermatheca ( Figure 2 View Figure 2 (c)) single, retort-shaped, length 48 μm (distorted).
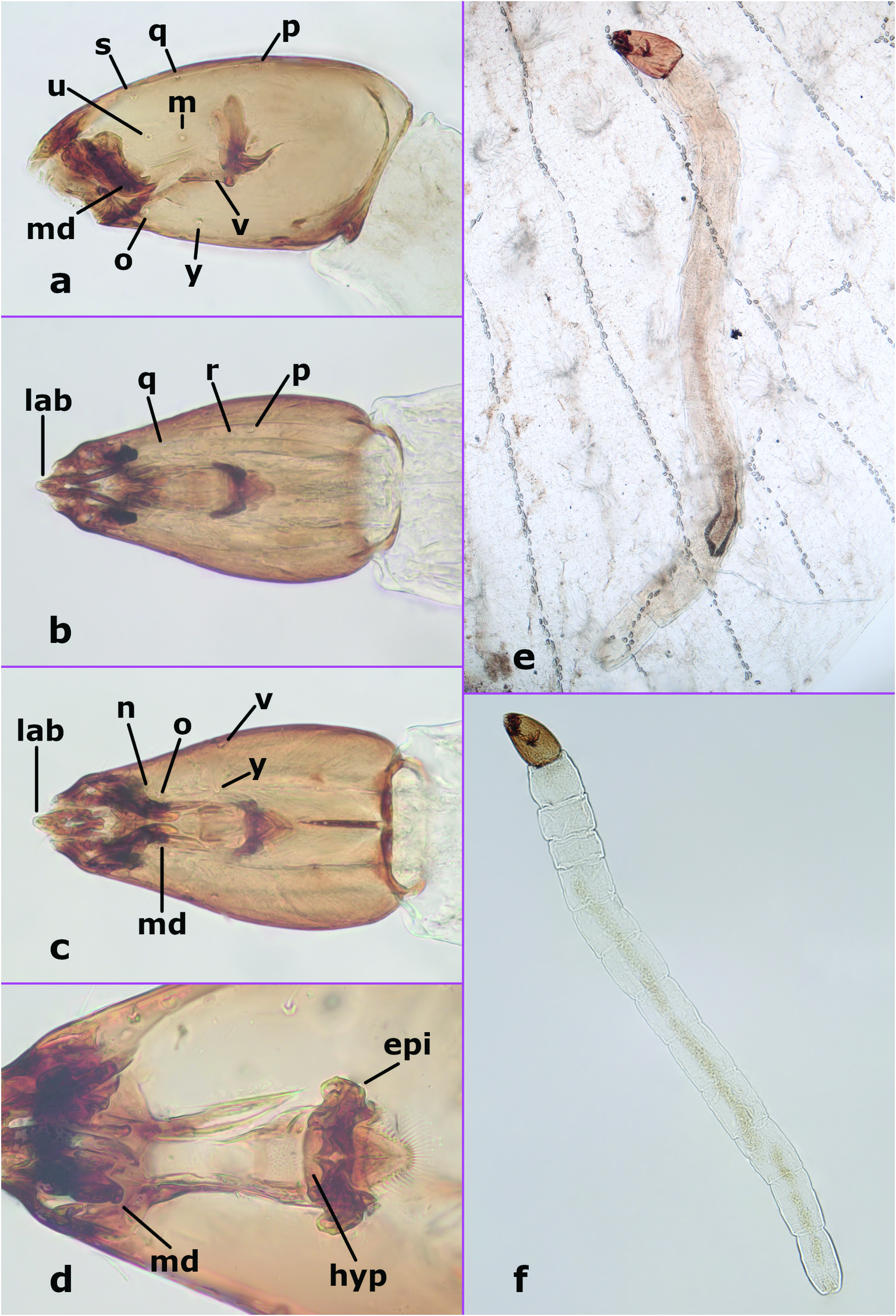
Fourth instar larva ( Figures 3 View Figure 3 (a–f), 8(b)). Total length 1.93–3.22 mm (n = 8). Colour in life whitish, with head capsule dark brown, length 0.27–0.35 mm (n = 18), L/ W 1.61 –1.92 (n = 7), tapering to apex; chaetotaxy as in Figures 3 View Figure 3 (a–c), unlabelled anterior setae are uncertain, j not visible in figures but present. Labrum ( Figures 3 View Figure 3 (b–c)) narrow, projecting anteriorly; anterolateral margins of head capsule extending anteriorly. Mandible ( Figures 3 View Figure 3 (a,c,d)) with 3 teeth, apical 2 teeth elongate, pointed, basal tooth broad. Epipharynx ( Figure 3 View Figure 3 (d)) massive, strongly sclerotised, lateral arms stout, short, dorsal comb with well-developed fringe. Hypopharynx ( Figure 3 View Figure 3 (d)) well developed. Caudal segment with approximately 5–7 well-developed hooks (on each side). Other details either not visible or similar to most other Dasyhelea ( Díaz et al. 2018, 2019).
Third instar larva ( Figure 8 View Figure 8 (a)). Similar to fourth instar. Total length 1.78–2.59 mm (n = 7). Colour in life whitish, with head capsule dark brown, length 0.20–0.23 mm (n = 8), L/ W 1.62 –1.74 (n = 4), tapering to apex.
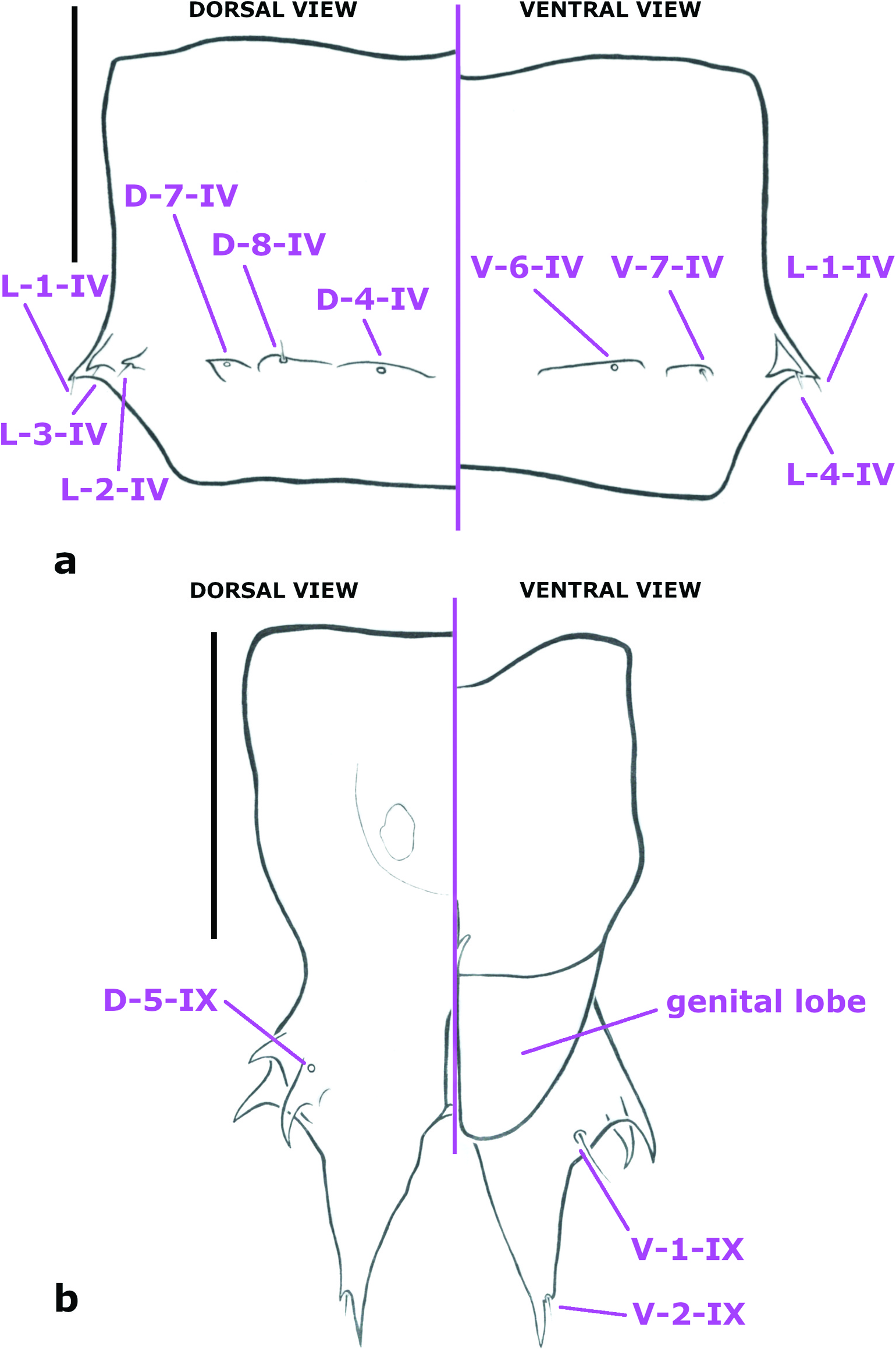
Pupa. Habitus as in Figures 4 View Figure 4 (a), 8(c–d). Total length 1.87–2.29 mm (n = 7). Clypeus ( Figure 6 View Figure 6 (a)) with CL-1-H, CL-2-H apparently absent in most, one specimen with very tiny seta CL-2-H, otherwise the area of these sensilla represented by small section of contorted cuticle; O-2-H absent; dorsal apotome ( Figure 6 View Figure 6 (b)) smooth, with DA-1-H a tiny seta, DA-2-H a campaniform sensillum. Mesonotum without short tubercles; respiratory organ ( Figure 4 View Figure 4 (b)) length/width 5.3–6.3 (n = 5), curved, apex pointed, somewhat circular in cross section, with a double row of about 7 circular pores each (so about 14 total), restricted to about apical 0.3 of respiratory organ, closely abutting apically, separated more basally, outer surface with strong annulations on posterior (inner curve) for about basal 0.2–0.5 to about 0.1–0.8, without spicules, tracheal tube curved along length of respiratory organ, somewhat thicker basally, with spirals restricted to base; sensilla: anterolaterals – 1 seta, 2 campaniform sensilla; dorsal setae ( Figure 6 View Figure 6 (c)) – D-1-T well anterior of D-2-T, D-3-T relatively close to D-2-T. Metathoracics ( Figure 6 View Figure 6 (d)) – 2 campaniform sensilla; M-2-T, M-3-T separated (not abutting), relatively near anterior margin of metathorax. Abdomen without dark pigmentation, segments 3–8 each with some wide, shelf-like tubercles; segment 9 with terminal processes closely approximated basally, each projecting posteriorly, each with 2–3 lateral, well-developed, pointed projections, with dorsal one bearing campaniform sensillum at its base; sensilla: tergite 1 ( Figure 6 View Figure 6 (d)) with 5 setae (D-2-I, D-3-I, L-1-I, L-2-I, L-3-I) and 2 campaniform sensilla (D-4-I, D-7-I); segment 4 ( Figure 7 View Figure 7 (a)) with D-2-IV absent; D-4-IV, D-8-IV each on wide, low, slightly separate tubercle, D-7-IV on slightly pointed tubercle; posterior dorsal sensilla in transverse row, arranged medially to laterally: D-4-IV, D-7-IV each a campaniform sensillum, D-8-IV a short seta on wide, low tubercle, L-1-IV, L-2-IV, L-3-IV, L-4-IV each a short seta on apically pointed tubercle; V-5-IV absent; V-6-IV a campaniform sensillum; V-7-IV a seta on wide, low tubercle; segment 9 ( Figures 4 View Figure 4 (c), 7(b)) long, terminal process with 3–4 divisions, laterally directed tubercles curved to somewhat hook-like, posteriorly directed portion elongate, tapering to point; with only one of D-5-IX present (a campaniform sensillum), D-6-IX apparently present (difficult to discern amidst shagreen), V-1-IX, V-2-IX elongate and short seta, respectively.
Distribution and bionomics
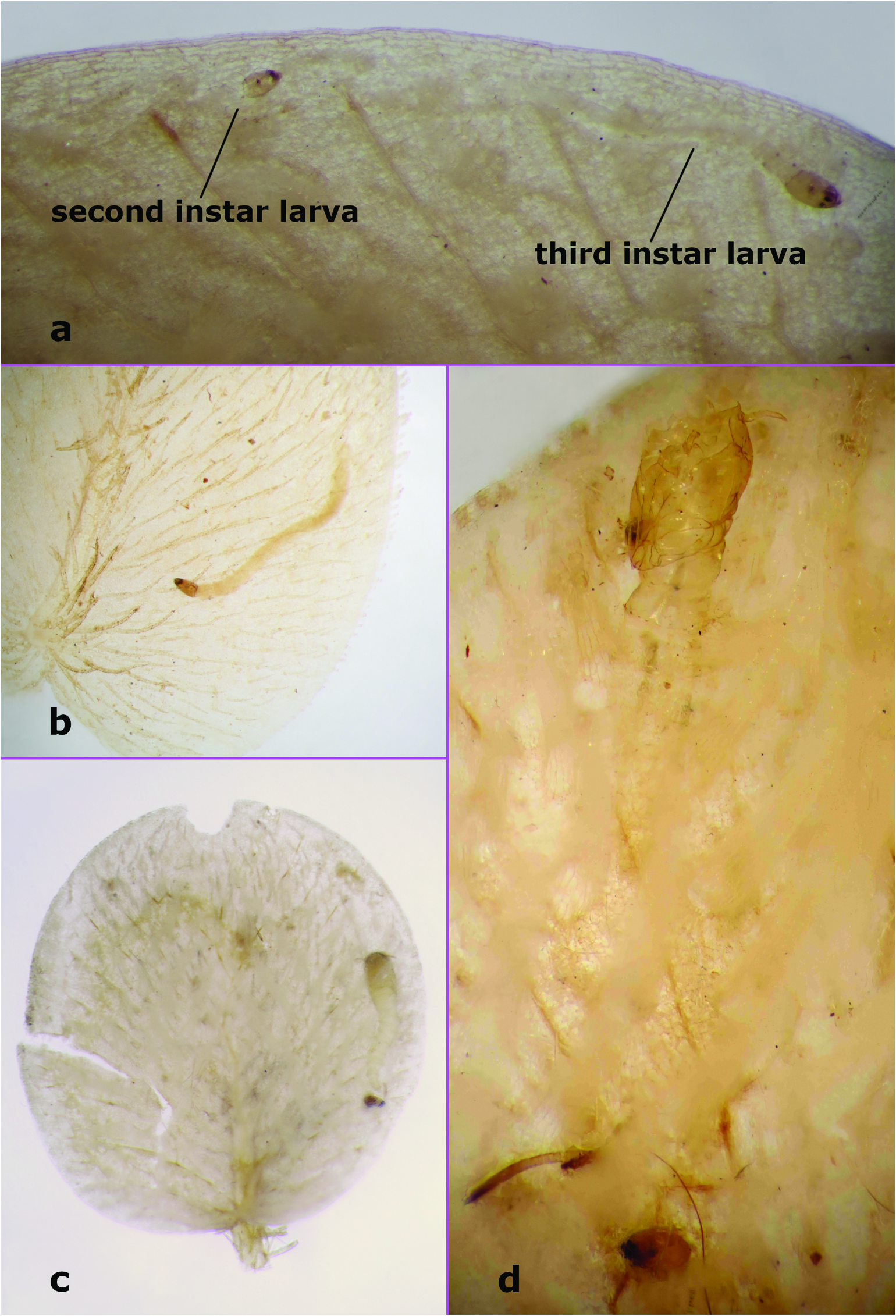
Dasyhelea mesophylla is known only from the type locality on the west coast of Costa Rica at an altitude of about 5 m. Adult specimens were reared from larvae and pupae present in S. minima leaves ( Figure 8 View Figure 8 ) floating in a lagoon on the northern margin of Carara National Park , about 1.5 km east from highway 34 along the hiking trail. The lagoon from which this species was collected in 1993 is periodically flooded by the abutting and extremely large Tárcoles River . The larvae and pupae were common but no specific data were taken in this regard. Virtually every clump of leaves had at least one leaf being mined (or that had been previously mined). Leaves were generally in clumps of 6–12 leaves. Borkent and Craig (2001) described the pupa of Stilobezzia rabelloi Lane , which have piercing respiratory organs to obtain oxygen from the dangling ̍roots′ (actually modified leaves), from the same habitat as D. mesophylla .
Observations of the mining larvae and of pupae in the S. minima leaves were made under the dissecting microscope as follows. Several fourth instar larvae were observed in some detail, although many were otherwise present. The larvae actively mined the leaves, scraping at live fern tissue, had green material in their guts and produced green excrement. The narrow labrum and anterolateral margins of head capsule extending anteriorly likely facilitate the scraping of plant tissue. The mines of at least fourth instar larvae produced a bulge on the upper surface of the leaves. The frass produced by larvae was either in the mine or on the surface of the leaf. At least some larvae periodically leave the mine for the surface and then burrow back into the leaf. A few larvae were observed to move from one leaf to another. Possibly this is a means of ensuring that overcrowding does not limit any given larva which can probably move from one abutting leaf to another. One larva was observed feeding on the interior of the leaf but with about 2/3 of its body on the surface of the leaf. Another larva was seen crawling on the surface, then gnawing through the surface to the interior and, after feeding for some time, drawing the rest of the body into the leaf. Generally, when a leaf was mined, there were only 1–2 fourth instars present (aside from other earlier instar larvae), but one instance of three larvae in a single leaf was observed.
Several third instar larvae were observed mining the interior of the leaves, in an identical manner to the fourth instar larvae, but no third instars were seen on the surface of the leaves. One second instar larva was observed in a tunnel just slightly wider than its body, winding through the leaf. The mines of third and fourth instar larvae were more excavated in places, connected by swollen (leaf surface bulging at least dorsally) tunnels about the width of those larvae. First and second instar larvae seemed to be concentrated on the peripheral margins of the leaves, while third and fourth instar larvae were generally in the main body of the leaves.
On the other hand, several leaves were observed to be partially mined but without larvae, suggesting the larva had died or, more likely, had moved on to another leaf to mine there.
Two larvae were observed to pupate. One pupa was entirely encased within the mine with no direct opening to the surface, other than the insertion of its respiratory organs through the surface cuticle of the leaf. Another pupa had cut an opening near the anterior end of the pupa, but without the respiratory organs protruding. Observed again later, the pupa had stuck one respiratory organ up through the leaf cuticle, indicating the pupa only needs to obtain oxygen periodically from the surface (or can withdraw for some time if disturbed). Both pupae had their fourth instar exuviae present nearby ( Figures 4 View Figure 4 (a), 8(c–d)). A number of other pupae were observed with either one or both respiratory organs protruding from the leaf.
Two emerging adults in the laboratory had free-floating pupal exuviae. The one pupa noted above that had been entirely encased by the surface of the leaf had made a jagged exit hole in the leaf, and the other appeared to escape through the hole already present. Some leaves were found with pupal exuviae with part of their abdomens still in the leaves.
It is worth noting that Forno and Bourne (1984) previously recognised an unnamed Dasyhelea as ̍phytophagous′ on Salvinia molesta D.S. Mitch. in Brazil. Another species, called Dasyhelea sp. 3 (grisea group), was reported by Torreias et al. (2013) from southeastern Brazil, where its ̍immature stages were collected in S. auriculata ′ Aubl (6). However, considering the sampling method used, the immatures (pupae only?) were most probably found among rhizomes and submerged, root-like leaves of Salvinia . Three males and one female were obtained but no details regarding their morphology are given by the authors ( Torreias et al. 2013). Pelli and Barbosa (1998) mentioned D. paulistana Forattini and Rabello as present on S. molesta in Brazil, but they did not assign this species to taxa feeding on Salvinia or to taxa causing harm to it. The latter Dasyhelea species together with D. pseudopollinosa Díaz and Ronderos were collected from mats of S. auriculata and Azolla filiculoides Lam. in Brazil and Argentina ( Díaz et al. 2014).
Taxonomic and phylogenetic discussion
This new species belongs to the grisea species group or subgenus D. ( Dasyhelea ) ( Dominiak 2012) if following the subgeneric division proposed by Remm (1962, 1979). The presence of the strongly modified pupal respiratory organ of D. mesophylla is shared by a number of species in the grisea species group, and because it is unique within the family ( Borkent 2014), we consider this a synapomorphy of these species. As such, from the limited number of Dasyhelea species known as pupae ( Borkent 2014), we consider D. mesophylla to form a monophyletic group with D. traverae Thomsen from eastern United States, D. pollinosa Wirth from the western and eastern United States, D. chani Wirth and Linley from Florida ( United States), D. paulistana from Argentina and Brazil, D. pseudopollinosa from Brazil, D. caesia Remm (syn. D. lugensis Brodskaya ) from Europe, and two unnamed species from Indonesia (Sumatra, listed as Holoconops sp. in Mayer 1934b) and Australia (A. Borkent, pers. obs.), respectively. All of them have virtually identical respiratory organs; these are thick at the base to about midlength and taper to a sharp apex. The respiratory organs of the pupae of D. mesophylla are somewhat more elongate than those of the other abovementioned species of Dasyhelea . Although not known as immatures, male D. unicolour Remm and D. stackelbergi Remm appear very similar to those in this group of species and may be closely related.
The pupae of D. mesophylla stick their respiratory organs through the upper surface of the mined S. minima leaves to obtain oxygen from the exposed surface of the leaf, suggesting that these other species also use their respiratory organs in a similar manner. However, because at least the larvae of D. traverae do not appear to mine leaves ( Thomsen 1935, 1937; Waugh and Wirth 1976) it is also possible that Dasyhelea species with such respiratory horns obtain air from water plants, similarly to S. rabelloi .
The piercing respiratory organs of pupae of a group of Stilobezzia Kieffer is clearly separately evolved, as evidenced by differences in the details of the respiratory organs compared with Dasyhelea as well as the phyletic distance between the two groups ( Borkent and Craig 2001).
In spite of the fact that species of Dasyhelea are common throughout much of the world, only 62 out of 628 known species have been described as larvae ( Díaz et al. 2013; Borkent 2014, 2016, 2018, 2019; Duan et al. 2019; Borkent and Dominiak 2020; Lu et al. 2020; Borkent et al. 2022). One reason is probably that larvae of Dasyhelea are generally morphologically conservative. However, the very narrow protruding labrum and corresponding anterolateral projections of the head capsule, likely important for scraping leaf material free to ingest, may be distinctive among those larvae which have been described so far, keeping in mind that, of the species with a piercing respiratory organ, only the larvae of D. caesia ( Brodskaya 1995) , D. chani ( Wirth and Linley 1990) and D. traverae ( Thomsen 1937) have been described. It is important to recognise that most descriptions of Dasyhelea larvae are rather superficial. Detailed comparative studies are needed of the significant differences in larvae between the numerous species of this genus. There are at least some marked differences exhibited by some taxa (eg those species living in Nepenthes pitcher plants in Southeast Asia which have extremely long, narrow head capsules; Tokunaga 1961; Wirth and Beaver 1979). None of the Dasyhelea species described from larvae or pupae other than D. mesophylla are known to be leaf-miners.
Types
Holotype: male adult with associated larval and pupal exuviae, on microscope slide, labelled ̍HOLOTYPE Dasyhelea mesophylla Dominiak and Borkent′, ̍ Costa Rica, 5 km NE, Tarcoles, C.R. 26-vii-1993, A. Borkent CD1490 , CD 1490′, ̍Reared from larva mining Salvinia leaf′ ( CNCI) . Allotype: female adult with associated pupal exuviae labelled as for holotype (CNCI). Paratypes: 3 males each with associated pupal exuviae, labelled as for holotype except ̍Reared from pupa embedded in Salvinia leaf′ (1, MNCR; 2, CNCI); 1 pupa, 19 fourth instars, 6 third instars, from type locality, 3 August 1993, in Salvinia leaves, CD1502 (4 fourth instars, MNCR; remainder, CNCI); 2 pupae and their associated fourth instar exuviae in Salvinia leaves, 2 fourth instars, 1 third instar from type locality, 3 August 1993, CD1502 (CNCI).
Derivation of specific epithet
The name mesophylla refers to the parenchyma between the epidermal layers of a leaf, the tissue where the larva and pupa of the new species have been found.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |