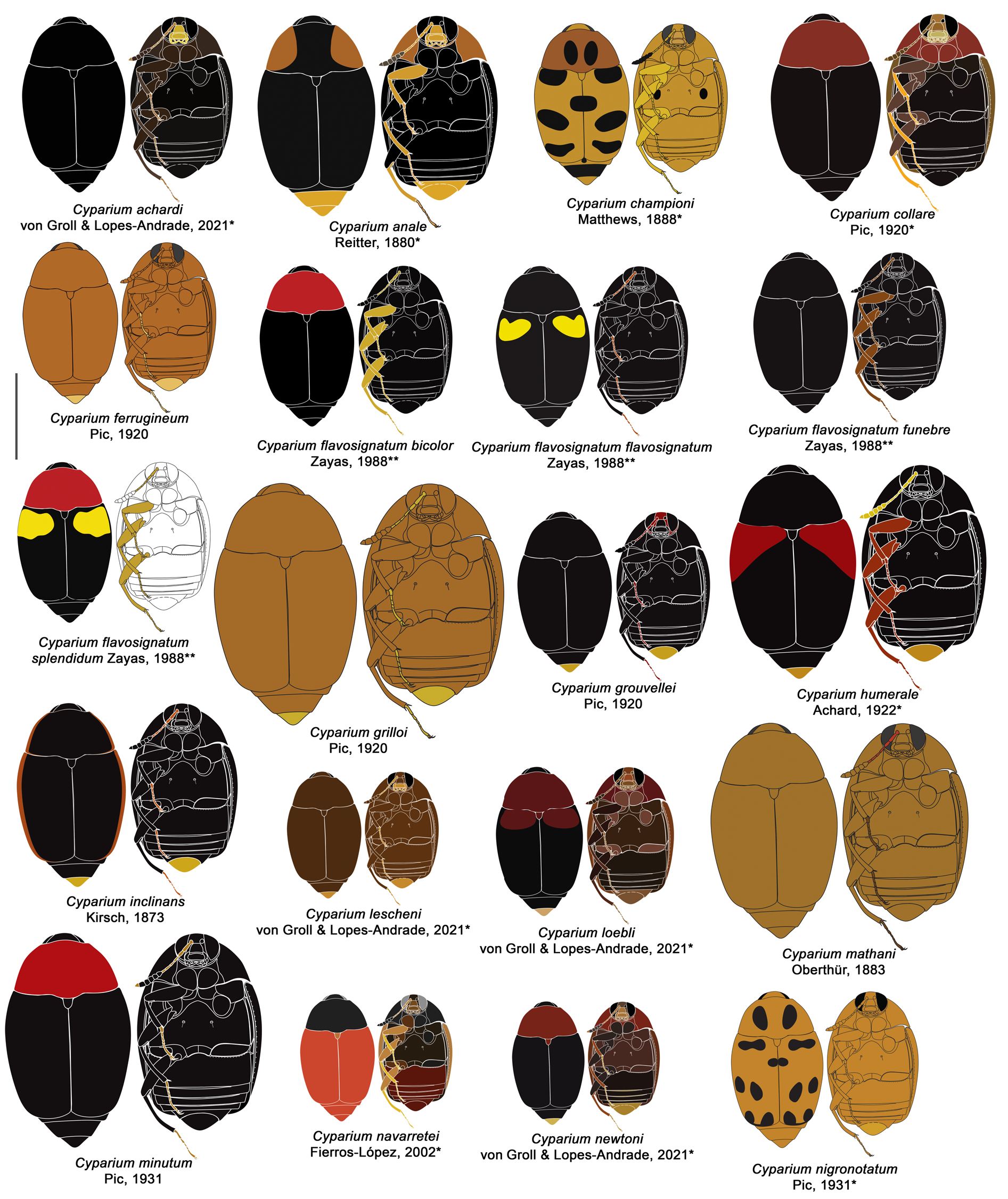
Cyparium collare Pic, 1920
|
publication ID |
https://doi.org/10.5852/ejt.2022.835.1909 |
|
publication LSID |
lsid:zoobank.org:pub:8B8432B1-C714-4179-8687-66902F4CBF53 |
|
DOI |
https://doi.org/10.5281/zenodo.7392081 |
|
persistent identifier |
https://treatment.plazi.org/id/A5415E5F-F458-FF9E-9855-FC08FC11FE09 |
|
treatment provided by |
Felipe |
|
scientific name |
Cyparium collare Pic, 1920 |
| status |
|
Cyparium collare Pic, 1920 View in CoL
Figs 4 View Fig , 47–55 View Fig View Fig View Fig View Fig View Fig View Fig View Fig View Fig View Fig ; Supp. file 2B, Supp. file 3 ( Figs 1A View Fig – 4H View Fig )
Cyparium collare Pic, 1920a: 4 View in CoL . Syntypes: Muséum national d’histoire naturelle (MNHN), Paris, France.
Material examined
BRAZIL – Sergipe • 3 ♂♂; Sta Luzia do Itanhi, Faz. Crasto ; 9–12 Sep. 1999; A. Bonaldo leg.; MCN 166400 (abdomen dissected), 166404, 166405 • 3 ♀♀; same collection data as for preceding; MCN 166398, 166401, 167484 • 2♂♂; Areia Branca, Est. Ecol.Serra de Itabaiana ; 14–20 Sep. 1999; A. Bonaldo leg.; MCN 166402, 166403 (abdomen dissected, preserved in glycerin) • 1 ♀; same collection data as for preceding; MCN 166406 . – Minas Gerais • 1 ♂; Viçosa, EPTEA Mata do Paraíso ; 8 Dec. 2014; I. PecciMaddalena leg.; CELC • 3 ♂♂, 5 ♀♀ ( 1 ♀, abdomen dissected, preserved in glycerin); same collection data as for preceding; 6 Dec. 2018; LabCol leg; CELC • 7 ♂♂, 3 ♀♀ ( 1 ♀ entirely dissected, preserved in glycerin; 1 ♂, 1 ♀, abdomen dissected, preserved in glycerin); same collection data as for preceding; 5 Nov. 2019; LabCol leg.; “Fungo 02 \\ Em Pleurotus pulmonarius ”; CELC • 1 ♀; same collection data as for preceding; “Fungo 03 \\ Em Mycena sp. ”; CELC • 14 ♂♂, 4 ♀♀ ( 1 ♂, 1 ♀ entirely dissected, preserved in glycerin; 1 ♂, 1 ♀, abdomen dissected, preserved in glycerin); same collection data as for preceding; “Fungo 01 \\ Em Xerocomus aff. brasiliensis ”; CELC • 9 ♂♂, 3 ♀♀ ( 3 ♂♂ entirely dissected, preserved in glycerin); same collection data as for preceding; 7 Nov. 2019; LabCol leg.; “Fungo 17”; CELC • 1 ♂; same collection data as for preceding; “Fungo 14 \\ Em Leucoagaricus rubrotinctus ”; CELC • 1 ♂, 1 ♀; same collection data as for preceding; “Fungo sem id”; CELC • 1 ♀; same collection data as for preceding; “Fungo A21 \\ Fotos: 712-713; 728-729, Em Heimiomyces neovelutipes ”; CELC • 1 ♀; same collection data as for preceding; “Fungo Que”; CELC • 1 ♂, 2 ♀♀; same collection data as for preceding; 12 Nov. 2019; LabCol leg.; “Fungo 09”; CELC • 2 ♀♀ ( 1 ♀, abdomen dissected, preserved in glycerin); same collection data as for preceding; 13 Nov. 2019; LabCol leg.; “Fungo 18 \\ Em Macrolepiota colombiana ”; CELC • 1 ♂; same collection data as for preceding; 14 Nov. 2019; “Fungo 22 \\ Em Marasmiellus sp. ”; CELC • 1 ♂; same collection data as for preceding; 19 Nov. 2019; “Fungo 18; Em Marasmiellus cubensis ”; CELC • 1 ♀; same collection data as for preceding; “Fungo 16 \\ Em Marasmiellus sp. ”; CELC • 1 ♀; same collection data as for preceding; “Fungo 22 \\ Em Leucoprinus ianthinus ”; CELC • 1 ♂, 2 ♀♀; same collection data as for preceding; “Fungo 13 \\ Em Marasmiellus cubensis ”; CELC • 2 ♀♀; same collection data as for preceding; “Fungo 05 \\ Em Marasmius sp. ”; CELC • 2 ♀♀; same collection data as for preceding; “Fungo 24 \\ Em Marasmiellus cubensis ”; CELC • 1 ♀; same collection data as for preceding; 21 Nov. 2019; LabCol leg.; “Fungo 38 \\ Em Marasmiellus sp. ”; CELC • 1 ♀; same collection data as for preceding; “Fungo 06 \\ Em Agaricus dulcidulus e Leucocoprinus brebissoni ”; CELC • 5 ♂♂, 2 ♀♀ ( 1 ♂, abdomen dissected, preserved in glycerin); same collection data as for preceding; “Fungo 26 \\ Em Lulesia lignicola ”; CELC • 2 ♀♀; same collection data as for preceding; “Fungo 16 \\ Em Marasmius araucariae ”; CELC • 2 ♂♂, 3 ♀♀ ( 1 ♀, abdomen dissected, preserved in glycerin); same collection data as for preceding; “Fungo 12 \\ Em Marasmiellus aff. ramealis ”; CELC • 1 ♀; same collection data as for preceding; “Fungo 52 \\ Em Marasmiellus sp. ”; CELC • 2 ♂♂, 1 ♀; same collection data as for preceding; “Fungo 41 \\ Em Lepiota sp. ”; CELC • 2 ♂♂; same collection data as for preceding; 26 Nov. 2019; LabCol leg.; “Fungo 23 \\ Em Marasmiellus cubensis ”; CELC • 1 ♂; same collection data as for preceding; “Fungo 18 \\ Em Entoloma ( Inocephalus) sp.”; CELC • 2 ♀♀ ( 1 ♀, abdomen dissected, preserved in glycerin); same collection data as for preceding; “Fungo 46 \\ Em Volvariella sp. ”; CELC • 1 ♂, 1 ♀; same collection data as for preceding; “Fungo 24 \\ Em Marasmius sp. ”; CELC • 1 ♀; same collection data as for preceding; “Fungo 19 \\ Pleteus sp.”; CELC • 1 ♂; same collection data as for preceding; “Fungo 37 \\ Conocybe sp. ”; CELC • 1 ♂; same collection data as for preceding; “Fungo 26 \\ Em Leucocoprinus sp. ”; CELC • 3 ♂♂; same collection data as for preceding; “Fungo 17”; CELC • 1 ♀; same collection data as for preceding; “Fungo 39 \\ Em Marasmius hematocephalus ”; CELC • 2 ♂, 1 ♀ ( 1 ♂ entirely dissected, preserved in glycerin); same collection data as for preceding; 28 Nov. 2019; LabCol leg.; “fotos 1103- 05 \\ Em Marasmius sp. ”; CELC • 1 ♂; same collection data as for preceding, “Trilha dos Gigantes”; 3 Feb. 2019; LabCol leg.; CELC • 1 ♀; Viçosa, Mata da Biologia ; 3 May 2014; S. Aloquio leg.; “\\ ex. Lepiota sp. ”; CELC • 2 ♂♂, 2 ♀♀; Viçosa, Recanto das Cigarras, Mata da Biol. ; 13 Nov. 2019; LabCol leg; “Fungo 11 \\ Em Coprinellus disseminatus ”; CELC • 1 ♂; Viçosa, Mata da Biologia ; 15 Oct. 2021; E. von Groll and A. Orsetti leg.; “Fungo 06”; CELC • 1 ♂, 1 ♀; same collection data as for preceding; “Fungo 26 \\ Em Leucocoprinus cepistipes ”; CELC • 1 ♂, 1 ♀; same collection data as for preceding; 23 Nov. 2021; E. von Groll and G.L.N. Martins leg.; “\\ Em Favolus tenuiculus ”; CELC • 8 ♂♂, 1 ♀; same collection data as for preceding; 26 Nov. 2021; “\\ Em Favolus tenuiculus ”; CELC .
Diagnosis
TL: 2.60–3.40 mm in males and 2.56–3.52 mm in females. Pronotum reddish brown, elytra black ( Fig. 47A View Fig ). Eyes remarkably wider than head, rounded ( Fig. 48A View Fig ). Antennomere XI hexagonal, longish ( Fig. 48B–C View Fig ). Hypomeron only laterally with imbricate microsculpture. Metaventrite smooth, but laterally with imbricate microsculpture. Tergite VIII of males coarsely punctate ( Fig. 52D View Fig ). Rounded ventral struts ( Fig. 52F View Fig ). Aedeagus with short apex; openings in dorsal view thin, forming a wide acute angle; parameres short ( Fig. 53A–C View Fig ). Distal gonocoxites curved and thick ( Fig. 54E View Fig ).
Redescription
COLORATION. Iridescent. Frons ochre, reddish brown; clypeus yellowish to ochre; mouthparts yellowbrown ( Fig. 48A View Fig , Supp. file 3 ( Figs 1D View Fig , 3D View Fig )); antennomeres I–VI and apex of XI yellow-brown; VII–X and basal part of XI darker ( Fig. 48B View Fig ). Pronotum and hypomeron reddish brown ( Fig. 47A–B View Fig , Supp. file 3 ( Figs 1E View Fig , 3E View Fig )). Scutellum reddish brown or black ( Fig. 49D View Fig ). Elytra black ( Fig. 47A View Fig ); epipleuron dark ochre. Mesanepisternum, metanepisternum and metepimeron dark ochre. Metaventrite dark brown, lighter laterally ( Fig. 47B View Fig ). Coxae and trochanters dark ochre; femora yellowish brown, apex yellow; tibiae and tarsi ochre ( Fig. 47B–C View Fig ). Tergite VI and VII black; tergite VIII yellow. Ventrites 1–4 dark brown; 5 and 6 yellow. Teneral specimens entirely light brown ( Fig. 47D–E View Fig ) or with pronotum lighter ( Fig. 47F View Fig ).
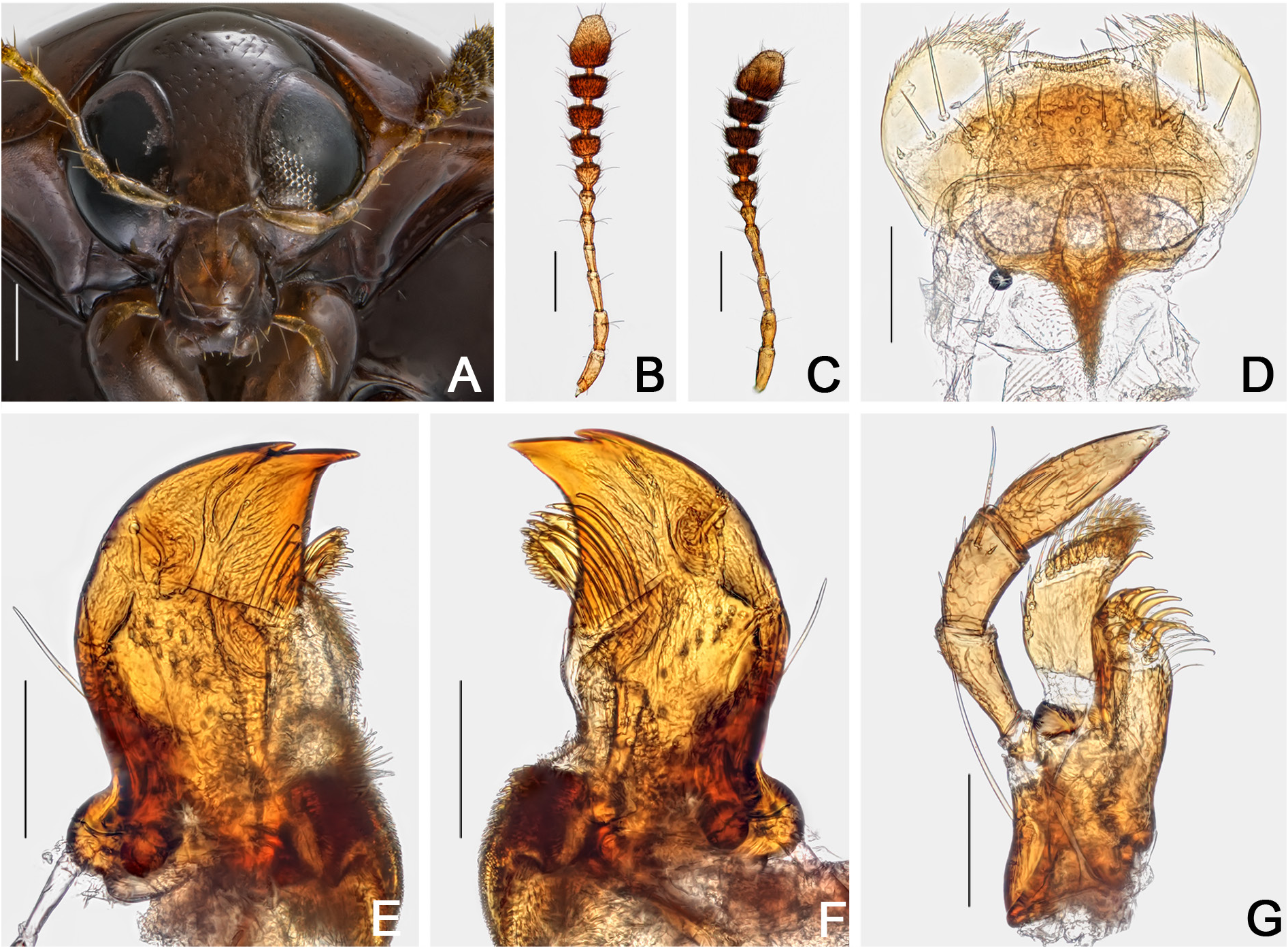
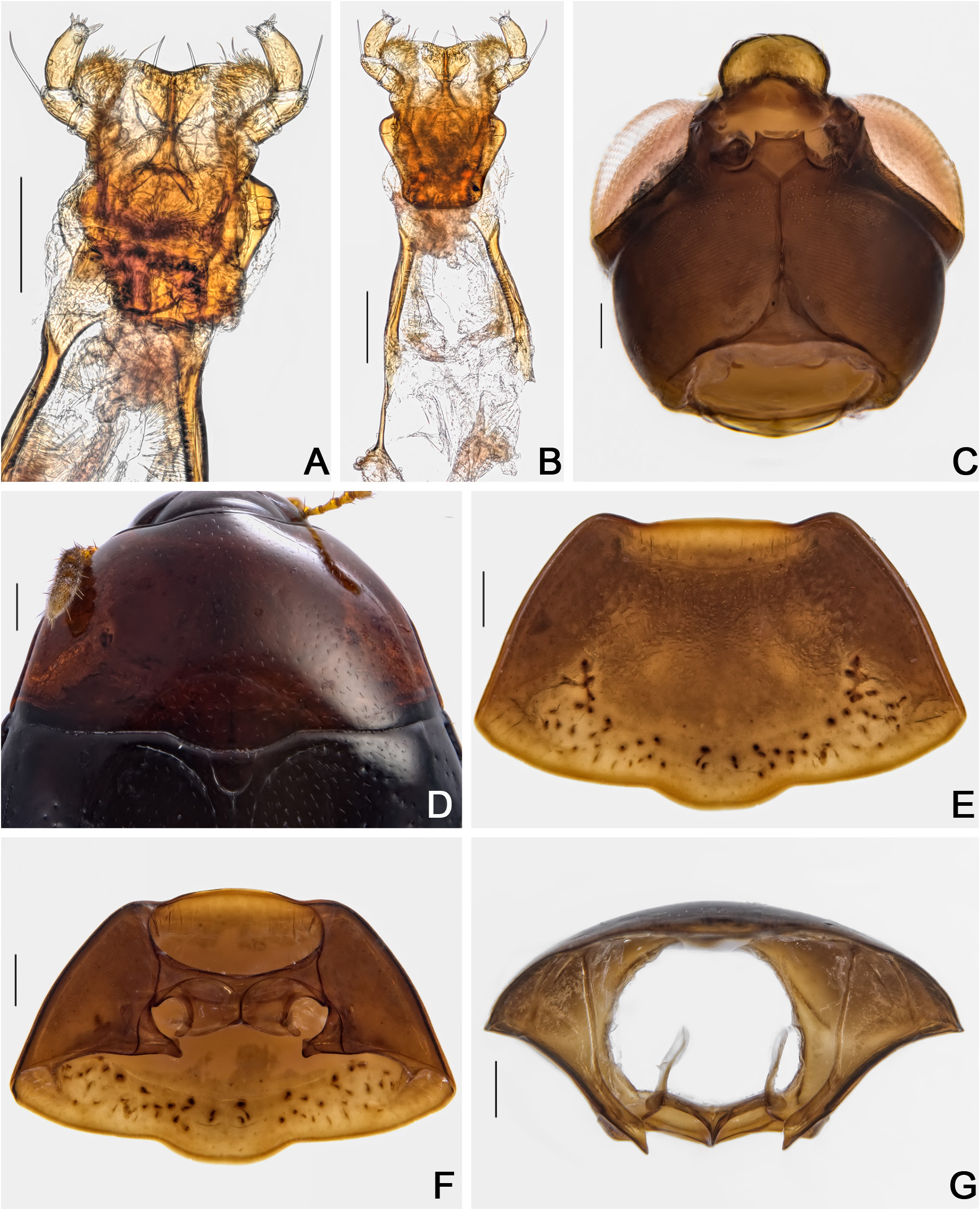
HEAD. Punctation dense, fine ( Fig. 48A View Fig ). Eyes remarkably wider than head, rounded ( Fig. 48A View Fig ). Labrum transverse, lateral areas rounded, not well marked at apex; central margin slightly curved; sclerotized portion rounded; lateral setae slightly exceeding margins of labrum; almost without pores centrally ( Fig. 48D View Fig ). Mandibles smoothly curved, subapical serrations on left mandible conspicuous ( Fig. 48E– F View Fig ). Maxillae with palpomere II long and thin; palpomere III somewhat globose; galea densely pubescent and lacinia moderately pubescent ( Fig. 48G View Fig ). Mentum laterally rounded, curved apically ( Fig. 49A View Fig ). Setae of labial palpomere II not exceeding palpomere III; palpomere III longish, with short apical setae ( Fig. 49A–B View Fig ). Post gena microsculptured with close transversal lines; gular pores limited to region next to eyes and submentum; gula triangular, small ( Fig. 49C View Fig ). Antennae not reaching or surpassing mesanepisternum, regardless of sex; antennal club smoothly delimited; antennomere XI hexagonal, longish ( Fig. 48B–C View Fig ).
PROTHORAX. Pronotum smooth, shining; punctation dense, fine; pubescence short, fine ( Fig. 49D View Fig ); transverse, sub-straight laterally, almost forming a right angle at posterior margin ( Fig. 49E View Fig ). Hypomeron laterally with imbricate microsculpture. Notosternal suture with an angle, outward directed ( Fig. 49F View Fig ). Profurca thin, short, not exceeding half of foramen ( Fig. 49G View Fig ). Prosternal process apically wavy ( Fig. 50A View Fig ).
MESOTHORAX. Mesonotum with prescutellar suture (= scutellar lines, Leschen & Löbl 2005) wavy ( Fig. 50B View Fig ). Scutellum rounded posteriorly ( Fig. 50B View Fig ). Anterior phragma thin and somewhat straight ( Fig. 50C View Fig ). Mesanepistenum with shallow imbricate microsculpture, inconspicuous in some. Procoxal rests triangular, straight posteriorly ( Fig. 50D View Fig ). Mesoventral and median lines strongly wavy; area between median and mesocoxal lines large ( Fig. 50D View Fig ). Mesoventral process curved, forming a wellmarked ridge ( Fig. 50E View Fig ).
METATHORAX. Metanotum with triangular alacrista, turned to the sides; scutoscutellar suture inconspicuous; median membranous area narrow and long ( Fig. 50F View Fig ). Metaventrite smooth, but with imbricate microsculpture laterally; punctation sparse, fine. Mesocoxal line forming an angle between coxal cavities; finely punctate under coxal cavities ( Fig. 50D View Fig ). Metanepisternum and metepimeron with imbricate microsculpture. Intercoxal plates smooth. Metendosternite with curved arms; ‘stalk ridge’ exceeding half length of stalk ( Fig. 51A View Fig ); ventral longitudinal flange wide and short in lateral view ( Fig. 51B View Fig ).
WINGS. Elytra slightly wider than long, partially covering tergite VI ( Fig. 47A View Fig ); basal ( Fig. 49D View Fig ) and sutural lines dashed and punctate; adsutural area with a row of setae, six rows of coarse punctures (not including sutural line) ( Figs 47A View Fig , 51C View Fig ); lateral lines punctate; apical coarse punctation dense; apical serrations moderately large, well visible ( Fig. 51D View Fig ); pubescence short, fine. Epipleuron with diffuse coarse punctures. Hind wings fully developed ( Fig. 51E View Fig ).
LEGS. Pro-, meso- and metacoxae, and femora with strigulate microsculpture. Femora somewhat straight ( Fig. 51F–H View Fig ). Pro- and mesofemora sparsely and coarsely punctate; metafemora sparsely and finely punctate. Mesotibiae densely spinose, spines fine ( Fig. 51G View Fig ). Metatibiae sparsely spinose, spines fine ( Fig. 51H View Fig ).
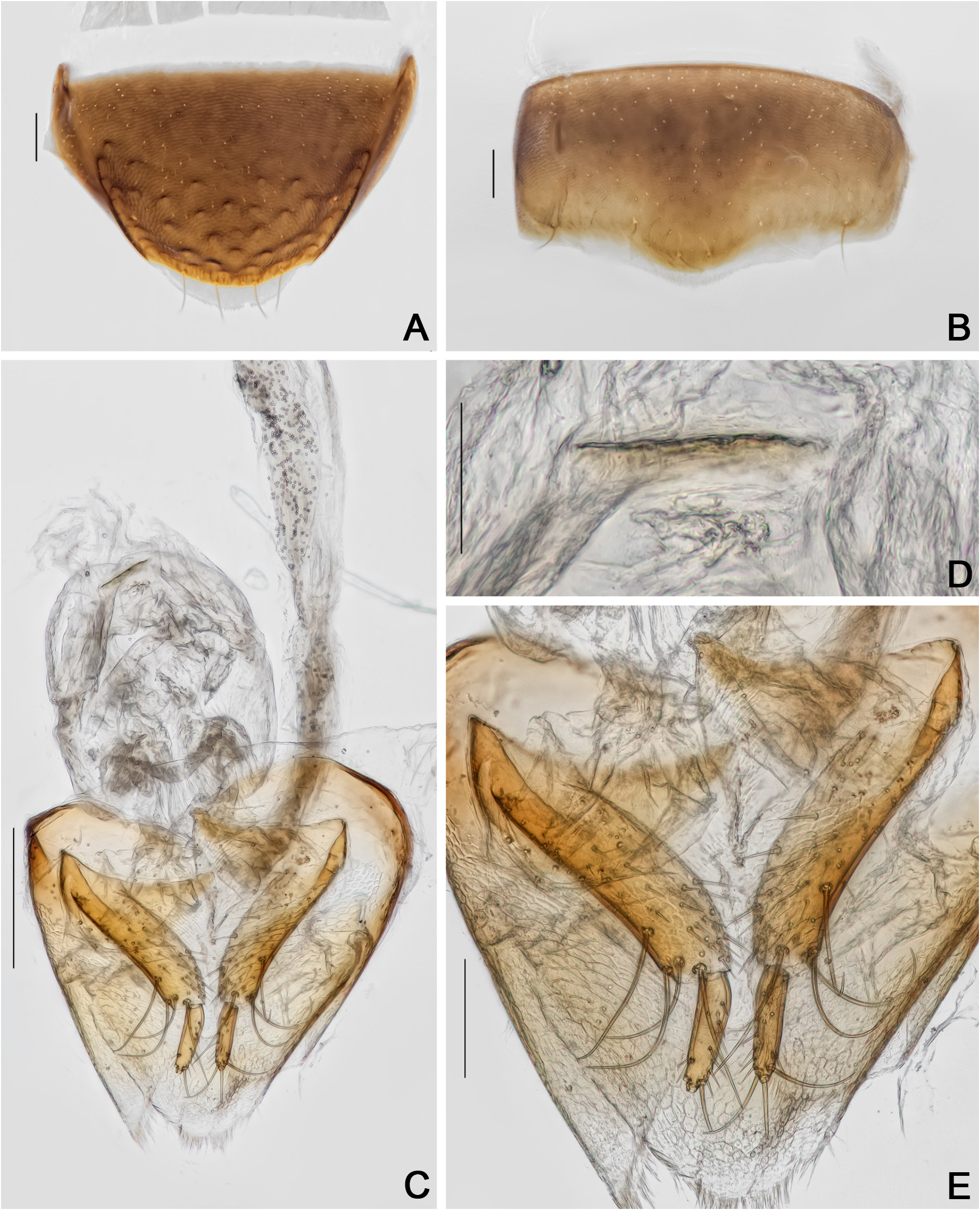
ABDOMEN. Tergites VI–VIII with imbricate microsculpture; tergite VII trapezoidal; punctation sparse, fine; pubescence dense, fine ( Fig. 52A View Fig ). Ventrites 1–5 ( Figs 52B, S View Fig 5F View Fig ) sparsely and coarsely punctate; pubescence sparse, fine; imbricate microsculpture anteriorly, and with strigulate microsculpture posteriorly ( Fig. 52C View Fig ). Metacoxal lines finely punctate.
Males
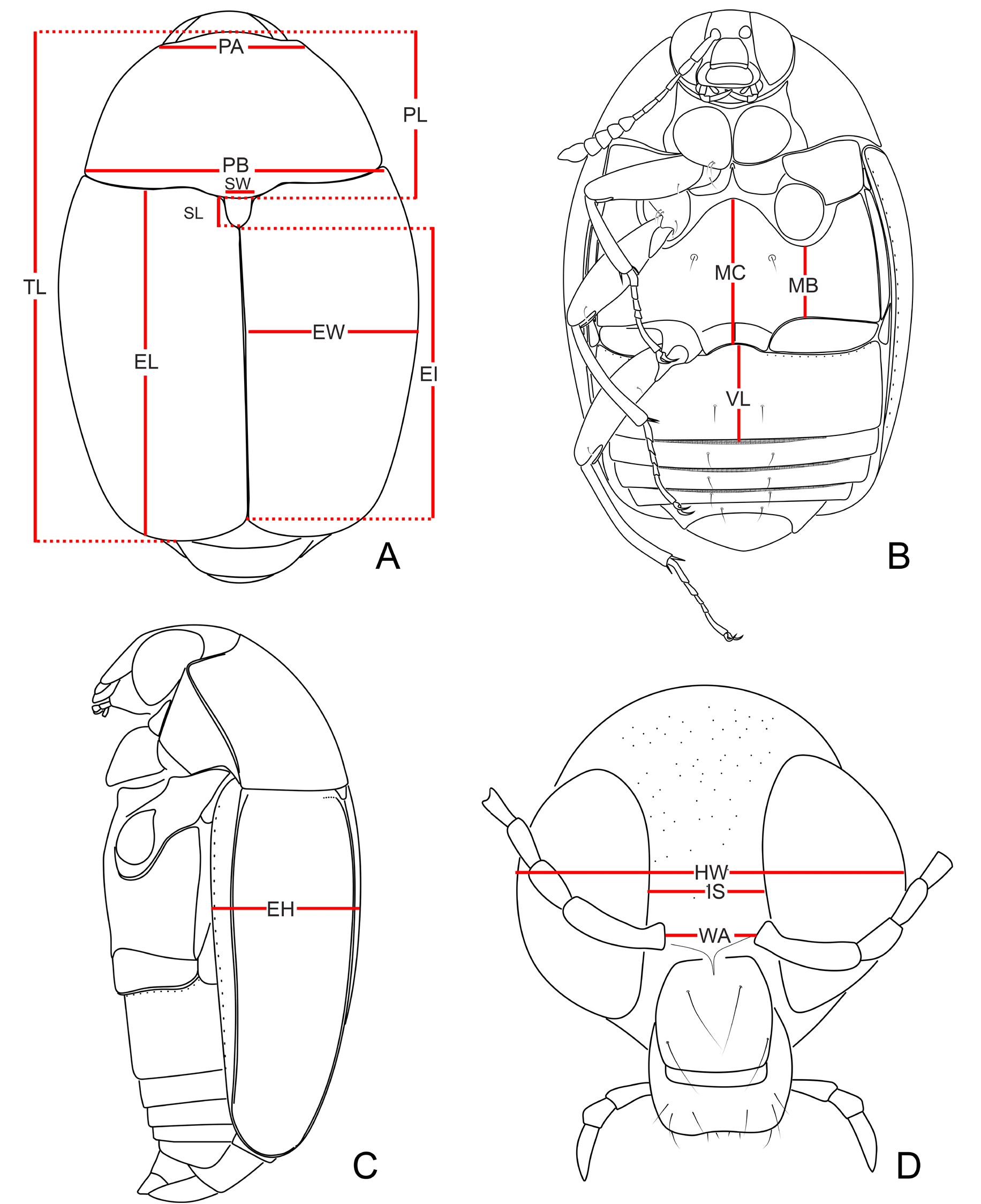
MEASUREMENTS (n = 1; in mm). Antennomeres (length(width): 0.15(0.06), 0.13(0.05), 0.14(0.04), 0.09(0.04), 0.11(0.05), 0.08(0.06), 0.07(0.08), 0.07(0.11), 0.08(0.12), 0.08(0.13), 0.18(0.14); (n = 15, unless otherwise specified; in mm): TL 2.60–3.72 (mean = 3.16, standard deviation ± 0.34), PL 0.90–1.35 (1.12 ± 0.12), PA 0.75–1.00 (0.88 ± 0.07), PB 1.50–2.25 (1.90 ± 0.23), SL (n =14) 0.13– 0.21 (0.16 ± 0.02), SW (n =14) 0.12–0.20 (0.16 ±0.02), EI 1.50–2.15 (1.84 ± 0.19), EL 1.77–2.55 (2.18 ± 0.23), EW 0.85–1.42 (1.12 ± 0.15), EH 0.30–0.95 (0.74 ± 0.20), HW 0.68–0.89 (0.79 ± 0.06), IS 0.15–0.25 (0.21 ± 0.02), WA 0.15–0.20 (0.18 ± 0.01), MC 0.70–1.15 (0.91 ± 0.12), MB 0.33–0.56 (0.44 ± 0.07), VL 0.46–0.75 (0.62 ± 0.08).
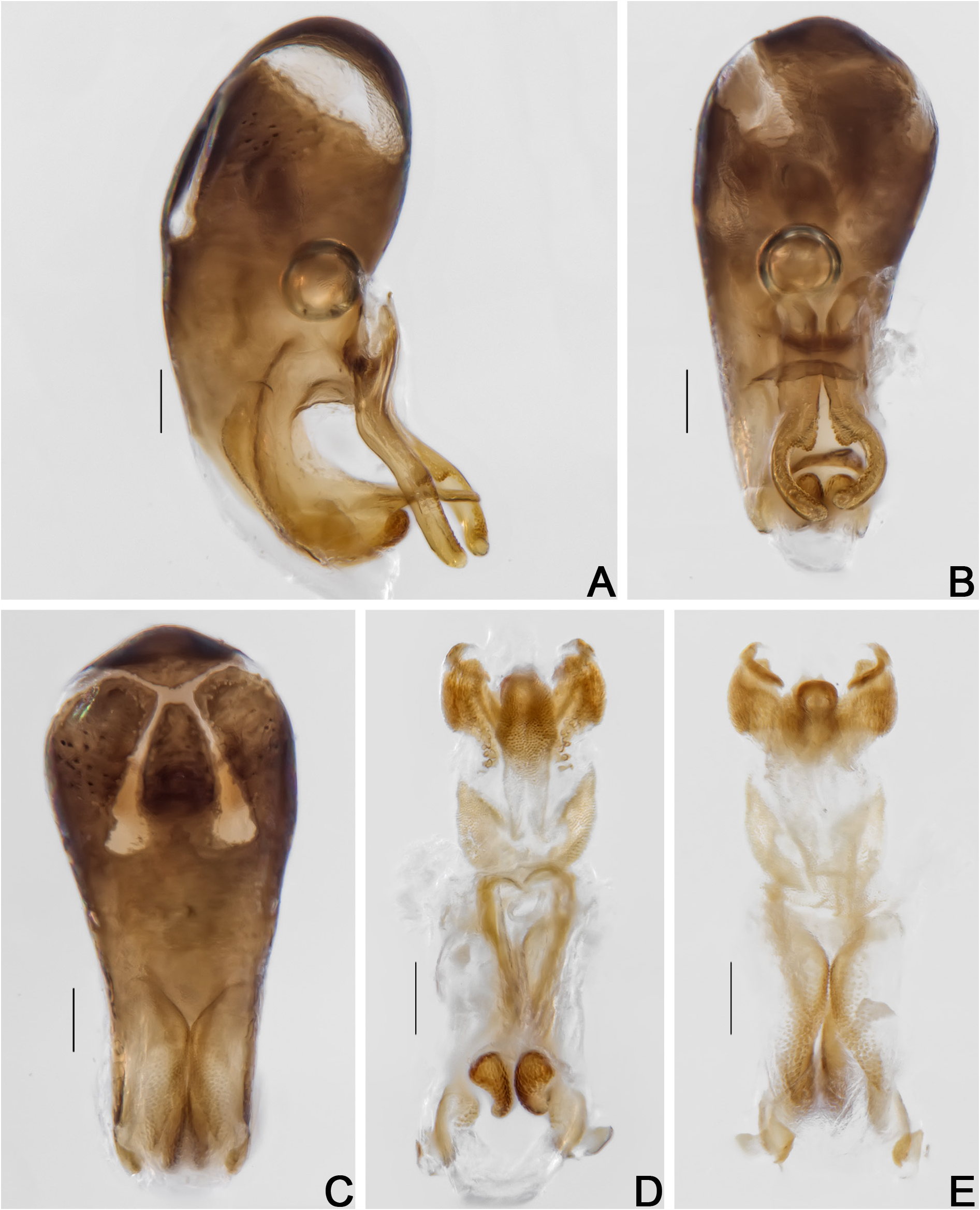
Pro- and mesotarsomeres I–III enlarged, with tenet setae ( Fig. 51F–G View Fig ). Tergite VIII triangular, acuminate posteriorly; punctation dense, coarse; subglabrous ( Fig. 52A, D View Fig , Supp. file 3 ( Figs 1F View Fig , 3G View Fig )). Tergite IX with rounded ventral struts ( Fig. 52E–F View Fig , Supp. file 3 ( Figs 1G View Fig , 4A–B View Fig )). Sternite VIII rectangular, short ( Fig. 52G View Fig ,Supp. file 3 ( Figs 1H View Fig , 4C View Fig )). Sternite IX more or less spoon-like ( Fig. 52F View Fig , Supp. file 3 ( Figs 1G View Fig , 4B View Fig )). Aedeagus sclerotized enlarged at base, apex of median lobe short; openings in dorsal view thin, forming a very acute angle ( Fig. 53A–C View Fig , Supp. file 3 ( Figs 2A–C View Fig , 4D–F View Fig )); internal sac with irregular sclerites, crown-like ( Fig. 53D–E View Fig ,Supp. file 3 ( Fig. 4G–H View Fig )); parameres short, curved and with denticular structures at base ( Fig. 53A–B View Fig , Supp. file 3 ( Figs 2A–B View Fig , 4D–E View Fig )).
Females
MEASUREMENTS (n = 1; in mm). Antennomeres (length(width): 0.16(0.06), 0.12(0.06), 0.13(0.04), 0.08(0.04), 0.10(0.05), 0.06(0.06), 0.06(0.08), 0.08(0.10), 0.07(0.12), 0.08(0.14), 018(0.15); (n = 14, unless otherwise specified; in mm): TL 2.56–3.64 (mean = 3.15, standard deviation ± 0.30), PL 0.92– 1.37 (1.14 ± 0.11), PA 0.77–1.02 (0.90 ± 0.06), PB 1.50–2.20 (1.92 ± 0.18), SL (n =12) 0.12–0.20 (0.16 ± 0.02), SW (n =12) 0.14–0.19 (0.16 ±0.01), EI (n =12) 1.52–2.12 (1.87 ± 0.16), EL (n =12) 1.85–2.52 (2.12 ± 0.19), EW 0.90–1.30 (1.14 ± 0.10), EH (n =12) 0.35–0.97 (0.75 ± 0.23), HW 0.65– 0.88 (0.79 ± 0.02), IS 0.20–0.26 (0.22 ± 0.02), WA 0.15–0.22 (0.18 ± 0.02), MC (n =13) 0.72–1.06 (0.93 ± 0.09), MB 0.35–0.95 (0.49 ± 0.14), VL 0.52–0.77 (0.65 ± 0.07).
Tergite VIII triangular, rounded posteriorly; punctation dense, coarse; subglabrous ( Fig. 54A View Fig ). Sternite VIII rectangular with a sooth projection; with strigulate microsculpture ( Fig. 54B View Fig ). Vagina and bursa copulatrix membranous without sclerites; vaginal plate with an apical sclerite, more or less short; oviduct bilobed, each lobe bearing a filiform spermatheca ( Fig. 54C–D View Fig , Supp. file 2B). Distal gonocoxites curved and thick; gonostyli long, slender ( Fig. 54C, E View Fig ).
Host fungi
Adults were collected from Marasmiellus cubensis (Berk. & M.A. Curtis) Singer (4 records, 8 individuals), Marasmiellus spp. (5, 6), Marasmiellus aff. ramealis (1, 5), cf. Marasmiellus sp. (yellow) (2, 5), cf. Pleteus sp. (1, 1), Marasmius araucariae Singer (1, 2), Marasmius haematocephalus (1, 1), Leucocoprinus cepistipes (1, 2), Leucocoprinus ianthinus (1, 1), Leucoprinus sp. (1, 1), Inocybe sp. ( Agaricales , Inocybaceae ) (1, 3), cf. Valvariela sp. (1, 2), Leucoagaricus rubrotinctus (Peck) Singer (1, 1), Lepiota sp. ( Agaricales , Agaricaceae ) (1, 3), Macrolepiota colombiana Franco-Mol. ( Agaricales , Agaricaceae ) (1, 2), Heimiomyces neovelutipes (Hongo) E.Horak ( Agaricales , Mycenaceae ) (1, 1), Lulesia lignicola B.E.Lechner & J.E.Wright (Agaricales) (1, 7), Pleurotus pulmonarius (Fr.) Quél. ( Agaricales , Pleurotaceae ) (2, 28), Volvariella sp. ( Agaricales , Pleurotaceae ) (1, 2), Pluteus sp. ( Agaricales , Pleurotaceae ) (1, 1), Mycena sp. ( Agaricales , Mycenaceae ) (1, 1), Entoloma ( Inocephalus) sp. (1, 1), Conocybe sp. ( Agaricales , Bolbitiaceae ) (1, 1), Coprinellus disseminatus (Pers.) J.E.Lange ( Agaricales , Psathyrellaceae ) (1, 4), unidentified mushrooms (1, 18) and Favolus tenuiculus P.Beauv. ( Polyporaceae , Polyporales ).
Remarks
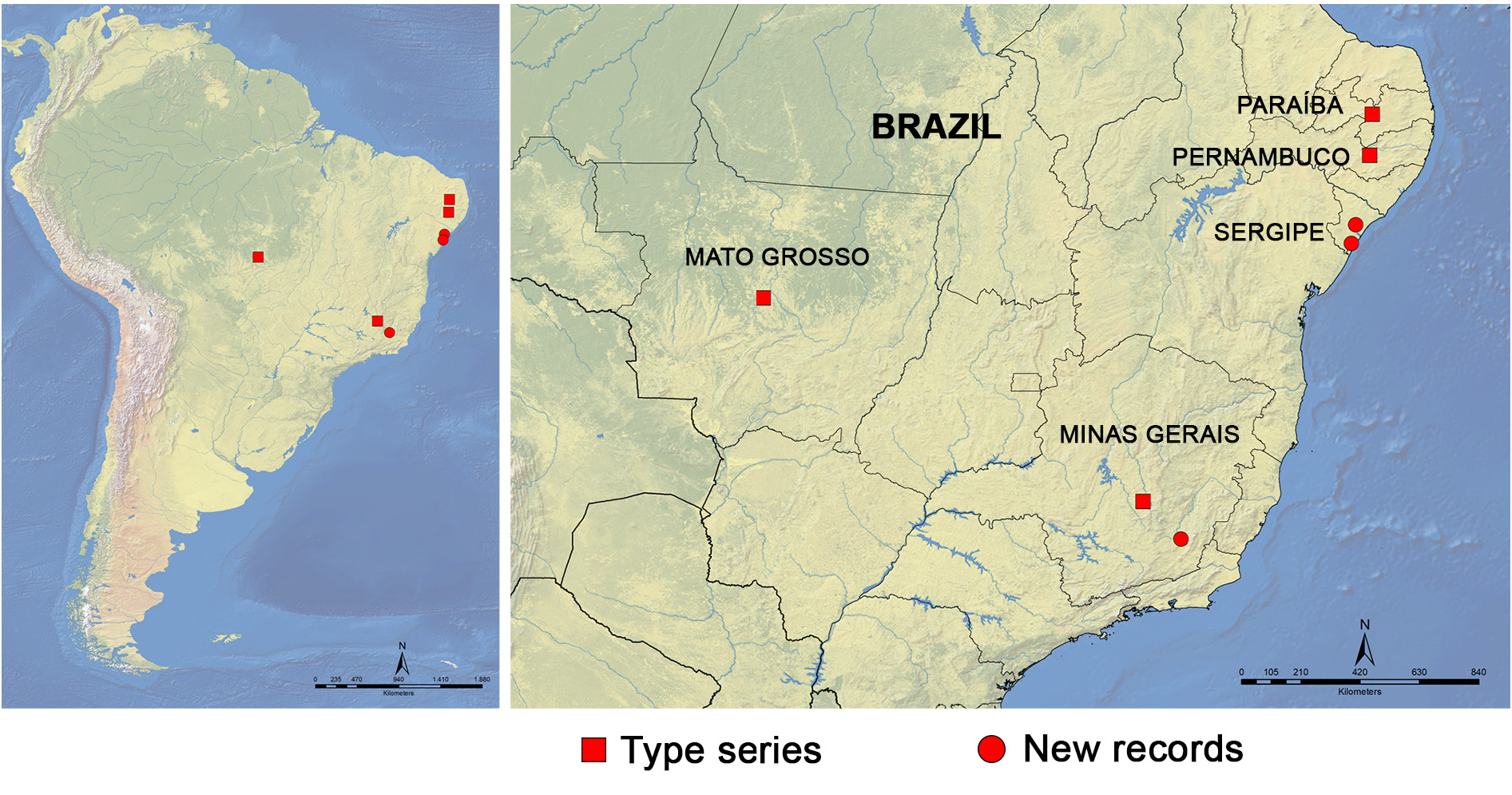
Images of the type series were requested from the MNHN, where these specimens were supposedly deposited ( Löbl 2018a), but we were informed that they could not find any specimens. Nonetheless, we decided to treat the specimens that we examined as C. collare because their morphology and localities fit the original description and the known distribution of the species. Fourteen individuals of different body length and collected from different fungi at Viçosa, one from Areia Branca (SE) (Supp. file 3 ( Figs 1–2 View Fig View Fig )), and another one from Sta Luzia do Itanhi (SE) (Supp. file 3 ( Figs 3–4 View Fig View Fig )) were dissected. No differences were found in the terminalia, including the flagellum. Furthermore, no external differences were observed. The total body length is quite variable ( 2.60–3.72 mm). The three individuals from Areia Branca (SE) are the largest (3.64, 3.68 and 3.72 mm), while individuals collected at Viçosa (MG) and Sta Luzia do Itanhi (SE) are in the same size range ( 2.56–3.52 mm).
Distribution
Known from Brazil [“Matu Sinhos, Minas”; “Matto Grosso”; “Pery-Pery, Pernambuco ”; “right bank of Parahyba ”]. New records from Areia Branca and Santa Luzia do Itanhi, state of Sergipe, Northeast, Brazil, and from “Mata da Biologia” and “EPTEA Mata do Paraíso”, campus of the Universidade Federal de Viçosa, Viçosa, Minas Gerais, Southeast Brazil ( Fig. 55 View Fig ).
| MCN |
Brazil, Rio Grande do Sul, Porto Alegre, Museu de Ciencias Naturais da Fundacao Zoo-Botanica do Rio Grande do Sul |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Scaphidiinae |
|
Tribe |
Cypariini |
|
Genus |
Cyparium collare Pic, 1920
| Groll, Elisa Von & Lopes-Andrade, Cristiano 2022 |
Cyparium collare
| Pic M. 1920: 4 |