Tanytarsus aries, Dantas & Hamada & Giłka, 2023
|
publication ID |
https://doi.org/10.11646/zootaxa.5271.1.4 |
|
publication LSID |
lsid:zoobank.org:pub:82D6F656-55DD-4DEB-84D8-BBB888E7B22E |
|
DOI |
https://doi.org/10.5281/zenodo.7864370 |
|
persistent identifier |
https://treatment.plazi.org/id/19965FA3-4F73-4F16-BAAC-9EC8C72608EA |
|
taxon LSID |
lsid:zoobank.org:act:19965FA3-4F73-4F16-BAAC-9EC8C72608EA |
|
treatment provided by |
Plazi |
|
scientific name |
Tanytarsus aries |
| status |
sp. nov. |
Tanytarsus aries View in CoL sp. nov.
https://zoobank.org/ urn:lsid:zoobank.org:act:
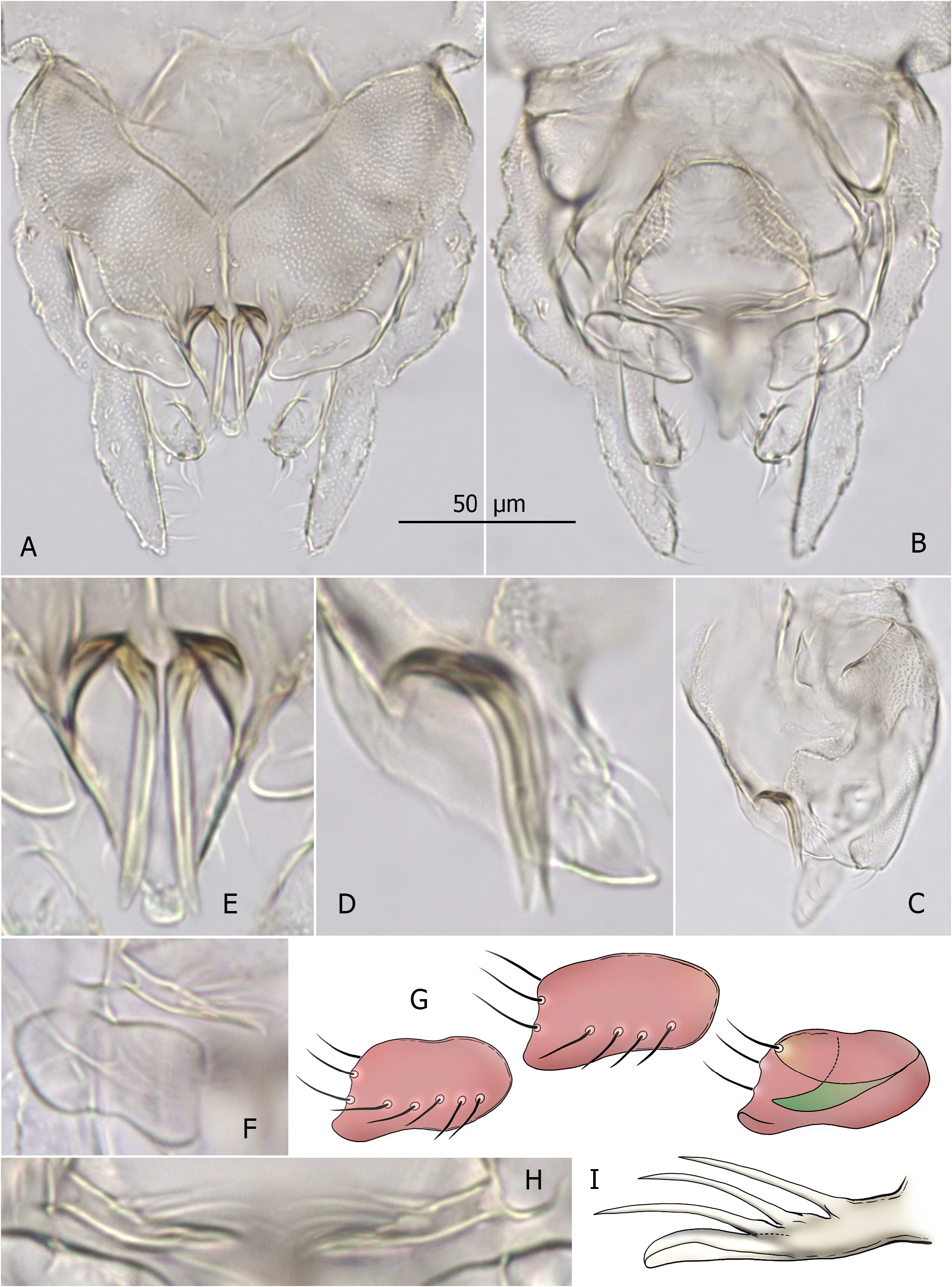
( Fig. 2 A–I View FIGURE 2 )
Type material. Holotype ♁, PERU, Cusco, Quincemil, Araza river tributary, 13º20′10′′S, 70º50′57′′W, 874 m a.s.l., 23–31.viii.2012, Malaise trap, J.A. Rafael, R. R. Cavichioli, D.M. Takiya ( MUSM) GoogleMaps . Paratypes: 5 ♁♁ (2 MUSM, 3 INPA), same data as holotype GoogleMaps .
Derivatio nominis. From Latin, in reference to the hypopygial anal point, in the lateral aspect resembling a horned head of a male sheep/ram ( Fig. 2D View FIGURE 2 ). Noun in apposition.
Diagnosis. AR ≤ 0.30. Tergite IX with microtrichia-free area near base of anal point, tergite bands Y-shaped. Anal point stout, triangular, crests broad, flanking large horn-like bars curved and turned up in proximal sections. Superior volsella subrectangular, with posteromedian corner slightly projected, bearing small ventral lip; digitus finger-like, not reaching margin of superior volsella. Median volsella with several setiform and single small foliate lamella. Inferior volsella with posteromedially directed head bearing dorsal flap.
Description. Adult male (n = 6)
Body size and proportions. Total length 2.05–2.21 mm. Wing length 1.04–1.07 mm. Total length/wing length 1.97–2.06. Wing length/length of profemur 2.12–2.30.
Colouration. Head capsule and palps yellow to light brown, eyes mostly pale brown, basal portion black, antenna brown. Scutal vittae and postnotum light brown, ground colour of thorax, scutellum, sternum, and haltere yellow to faint brown. Legs yellow to light brown. Wing veins yellow, membrane pale. Abdomen yellowish.
Head. Eyes bare, with well-developed dorsomedian extensions. Antenna with 13 flagellomeres; ultimate flagellomere 122–125 μm long; AR 0.28–0.30. Frontal tubercles 7–8 μm long. Tentorium 93–100 μm long, with elongate digitiform apex. Temporal setae 7–9 on each side. Clypeus with 10–13 setae. Lengths of palpomeres 1–5 (in μm): 20–25, 23–26, 78–85, 92–94, 143; third palpomere with 2 sensilla clavata subapically, 12 μm long.
Thorax. Ac 14–18, restricted to anterior region of scutum; Dc 5–6 on each side, uniserial; Pa 1 on each side; Scts 4. Scutum projected and rounded anteriorly, overreaching antepronotum.
Wing. Obovate, with anal lobe strongly reduced. Almost all veins (except subcosta) and entire membrane posterior to radial veins area (except 1/5 basal of m and cu cells) covered with macrotrichia. Brachiolum with 1 seta. VRCu 1.40–1.46.
Legs. Foreleg tibia with short lanceolate spur 16–19 μm long. Tibial combs of mid and hind legs separated; spurs of mid leg unequal: one apically curved, 20–22 μm long, second straight, 12–16 μm long; spurs of hind leg unequal: one apically curved, 25–26 μm long, second straight, 16–17 μm long. Basitarsus of mid leg without sensilla chaetica. Lengths and proportions of legs as in Table 1.
Hypopygium. Tergite IX covered with dense short microtrichia except for bare area near base of anal point, with two simple median setae; lateral teeth small, bilobed; tergite bands Y-shaped, fused part ~20–25 μm long, reaching anal point base ( Fig. 2A View FIGURE 2 ). Anal point stout, triangular, lateral margins with 3–4 setae, crests broad and round, flanking large (27–33 μm long) horn-like bars—strongly curved and turned up in proximal sections ( Fig. 2A, C–E View FIGURE 2 ). Superior volsella 26–29 μm long, subrectangular, posteromedian corner slightly projected, with small ventral lip; 4–5 setae dorsally, 2 setae on median margin and 1 seta on anteroventral tubercle, microtrichia on dorsal surface absent; digitus finger-like, 12–14 μm long, not reaching median margin of superior volsella ( Fig. 2A, B, F, G View FIGURE 2 ). Stem of median volsella simple, 12–13 μm long, with three setiform and one small foliate lamella ( Fig. 2B, F, H, I View FIGURE 2 ). Inferior volsella 45–52 μm long, slightly curved, with posteromedially directed head bearing dorsal flap ( Fig. 2A, B View FIGURE 2 ). Phallapodeme 50–56 μm long; transverse sternapodeme 35–38 μm long, with small oral projections. Gonocoxite 75–82 μm long. Gonostylus 52–55 μm long, slightly swollen at mid length, tapering to slender tip. HR 1.38–1.58, HV 3.90–4.25.
Female and immature stages. Unknown.
Taxonomy. Säwedal (1981) proposed Caladomyia for 18 species known at that time, which have been divided into two groups, spixi and orellanai. The division has been, however, considered unwarranted ( Reiff 2000) and ceased to be used ( Trivinho-Strixino 2012). Moreover, difficulties in diagnosing and delimiting or remarks on close relations between Caladomyia and Tanytarsus have been raised (e.g., Reiff 2000, Sanseverino 2006, Trivinho-Strixino 2012), also based on the fossil record ( Zakrzewska & Giłka 2013). Recently, after synonymizing Caladomyia and Tanytarsus , all former Caladomyia have been proposed to be placed in the ortoni group, recognized as monophyletic, although DNA sequences of only three described and named species (among 30) + those of two specimens of unknown Caladomyia have been used in the molecular analysis ( Lin et al. 2018). Phylogenetic relationships within these species still need support using integrative methods, those of molecular, based on at least the majority of described species, and their morphology. The significant heteromorphism in former Caladomyia seems to reflect the full range of structural diversity found in Tanytarsus , from relatively simple to the most sophisticated, thus the concept of one group for all these species can be perceived as tentative. Hence, we do not include T. aries to the ortoni group and refrain from proposing a possible division in the cluster(s) of these taxa (not an aim of this study), but we present the new species that extends the knowledge on the structural diversity. Regarding the shape of the gonostylus, anal tergite and volsellae, T. aries slightly resembles T. humboldti ( Säwedal, 1981) . However, it differs from all former Caladomyia and other Tanytarsus in the shape of the anal point, bearing broad crests and large, strongly curved horn-like bars ( Fig. 2 View FIGURE 2 ). The low antennal ratio (AR 0.3 or less) is a supplementation of the diagnosis for T. aries .
Geographical distribution and bionomics. Tanytarsus aries is known only from the type locality in the highlands of Amazonian Forest in Peru ( Fig. 1A, B View FIGURE 1 ). All specimens were collected using a Malaise trap set over a small rocky-bottomed stream surrounded by dense vegetation. This region is known for its numerous long and narrow valleys, mountain streams and warm, humid, and rainy weather ( Pulgar-Vidal 1996, Brack & Mendiola 2004). As noted by Brack & Mendiola (2004), this ecoregion is a significant centre of endemism; however, it has been rapidly degraded by human activities, particularly those related to occupation along roads.
| R |
Departamento de Geologia, Universidad de Chile |
| INPA |
Instituto Nacional de Pesquisas da Amazonia |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Chironominae |
|
Tribe |
Tanytarsini |
|
Genus |