Phyllospadix torreyi (Zapata and McMillan, 1979)
|
publication ID |
https://doi.org/10.1016/j.phytochem.2022.113256 |
|
DOI |
https://doi.org/10.5281/zenodo.8257555 |
|
persistent identifier |
https://treatment.plazi.org/id/A2143D69-FFE9-FFD7-FC89-F9075ABFFDCD |
|
treatment provided by |
Felipe |
|
scientific name |
Phyllospadix torreyi |
| status |
|
2.1. Determination of the phenolic fingerprints of P. torreyi View in CoL View at ENA by HPLC-DAD and LC/MS
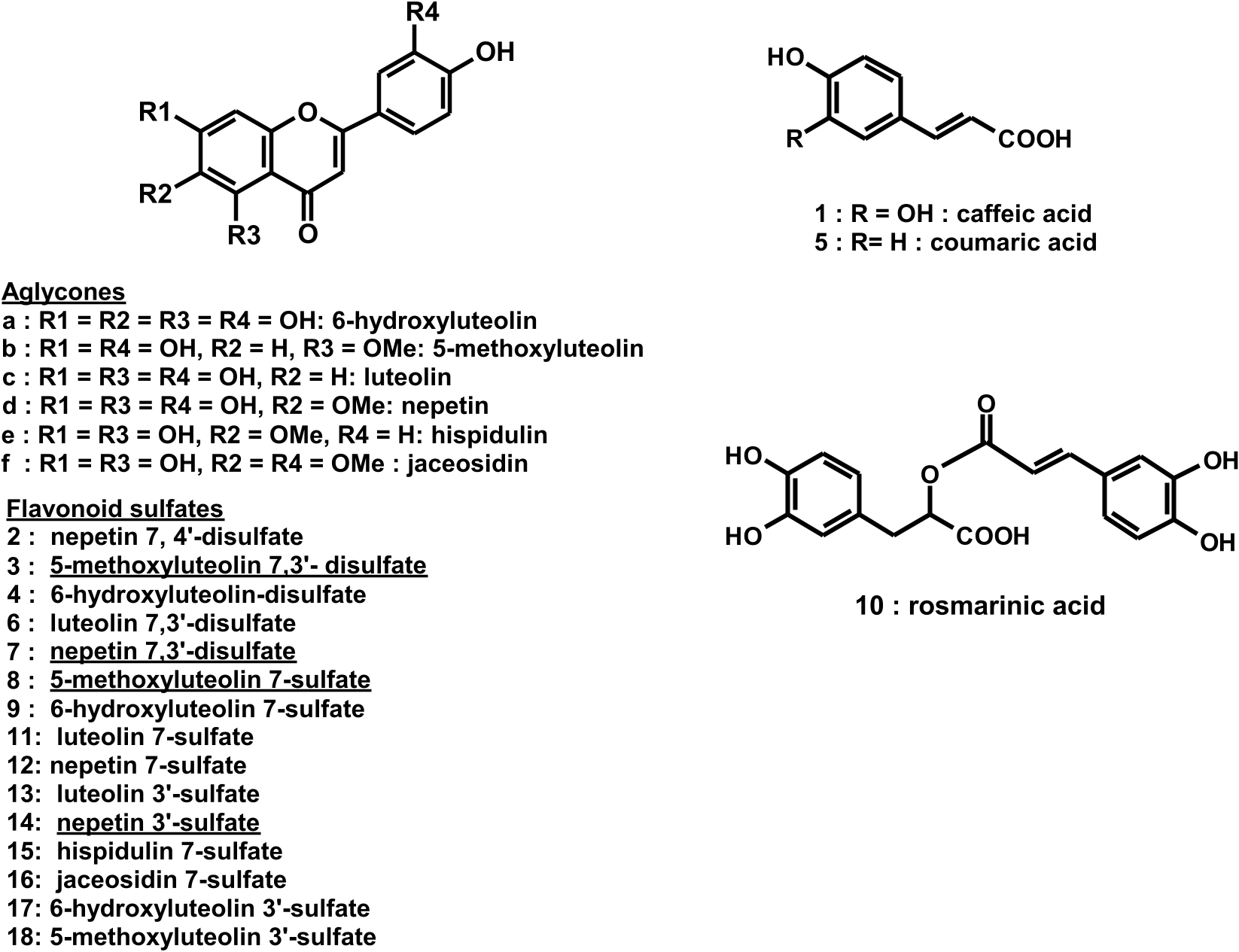
The crude extracts of P. torreyi were analyzed by HPLC-DAD and HPLC/ESI-MS in positive mode to obtain their chromatographic profiles, on-line UV spectra and mass spectral information regarding their components. Sixteen peaks of variable intensity were detected ( Table 1, Fig. 2). Comparison of retention time and UV spectra with those of flavonoid standards indicated the absence of flavonols and flavanones. Examination of on-line UV spectra enabled the identification of three phenolic acids and thirteen flavonoids (Fig. S31). The phenolic acids were identified as caffeic- ( 1, Rt 12.1 min), coumaric- ( 5, Rt 16.7 min), and rosmarinic acid ( 10, Rt 23.2 min), of which rosmarinic acid was predominant (Figs. 2 and 3). The flavonoid pattern was largely dominated by three flavonoids ( 3, 6 and 7), along with compounds in moderate amounts ( 8, 11–16), or in low to trace amounts ( 2, 4, 9).
– 6
Total acid hydrolysis of the crude extract resulted in the complete disappearance of the 13 peaks assigned as flavonoid ( 2–4, 6–9 and 11–16), and the appearance of six aglycones ( a -f, Fig. 2). Peaks assigned to phenolic acids ( 1, 5, 10) were recovered unchanged. Based on their UV spectra and comparison to standards and data from literature, the aglycones were identified as 6-hydroxyluteolin ( a, Rt, 24.1 min), 5- methoxyluteolin ( b, 25.1 min), luteolin ( c, 30.2 min), nepetin ( d, 30.9 min), hispidulin ( e, 33.6 min), and jaceosidin ( f, 34.0 min). This order of elution on C18 column and UV λ max values of these aglycones are in agreement with literature data ( Bojilov et al., 2017; Greenham et al., 2003). It should be noted that whilst the introduction of a methoxyl group generally makes the product more lipophilic, resulting in a higher retention time, this is not the case for 5-methoxyluteolin, which was eluted earlier than luteolin due to the absence of internal hydrogen bond with the carbonyl group ( Greenham et al., 2003). 5-Methoxyluteolin, luteolin, nepetin, hispidulin were unambiguously assigned by spectroscopic data (NMR, UV, and MS). Elution order and on-line UV spectra for 6-hydroxyluteolin and jaceosidin were in agreement with literature data ( Bojilov et al., 2017; Greenham et al., 2003; Tom´as-Barber´an et al., 1987). Controlled hydrolysis in mild condition released flavonoids 13 and 14, whose peak intensity has increased, along with two additional compounds ( 17 and 18), and the six aglycones a -f (Figs. 2 and 3, and S31). This suggests that compounds 13–14 and 17–18 are intermediates with respect to the level of sulfation of their respective flavonoid aglycone.
2.2 View Table 2 . Purification of the extracts and structure identification of flavonoid compounds
The crude aqueous-methanolic extract was serially partitioned between water and organic solvent of increasing polarity, i.e. methylene chloride (F1, 0.2% yield), ethyl acetate (F2, 0.6% yield), and then nbutanol (F3, 11.2% yield). Of these, only F3 was found to contain significant amounts of flavonoid. HPLC analysis of F2 showed a complex mixture of apolar products dominated by 3, 4-dihydroxy benzaldehyde (5% of the total), caffeic acid (18%), coumaric acid (7%) and rosmarinic acid (13%). They were identified by UV, comparison to authentic standards, and NMR. This is the first report of rosmarinic acid from the genus Phyllospadix . The presence of caffeic- and coumaric acid in P. torreyi was previously reported by Zapata and McMillan (1979).
Fractionation of F3 by successive chromatography on C18 reverse phase silica gel led to the isolation of seven pure flavonoids ( 3, 6–7, 11–14), of which 3, 7, and 14 were previously undescribed.
Compound 3 eluted at 14.4 min in the chromatographic run. The positive ESI-MS spectrum gave a quasimolecular peak [M+H] + at 461 m /z, which was compatible with the molecular formula C 16 H 12 O 12 S 2. Two pairs of another significant ions at m/z 403 ([M-80 +Na] +, 381 [M-80 + H] +, and 323 [M-160 + Na] +, 301 [M-160 + H] + confirmed the presence of two sulfate groups. The 13 C NMR spectrum in DMSO‑ d 6 showed 16 resonances (1 methoxy, 6 CH, and 9 quaternary C including a carbonyl). The 1 H NMR spectrum showed six proton signals in the aromatic region in accordance with a luteolin derivative, i.e. a pair of meta coupled protons at δ 6.70 (1 H, d, J =2.2 Hz, H-6) and δ 7.12 (1 H, d, J = 2.2 Hz, H-8), a one proton singlet at δ 6.50 (H-3), and an AMX spin system at δ 7.84 (1 H, d, J =2.3 Hz, H-2 ′), δ 7.63 (1 H, dd, J =2.3, 8.5 Hz, H-6 ′), δ 6.97 (1 H, d, J = 8.5 Hz, H-5 ′) ( Table 2 View Table 2 ). Resonances of carbon and proton showed the typical ipso and ortho shifts due to the presence of a sulfate group at position 7 (strong deshielding of H-8 and C-8; shielding of C-7), and 3’ (strong deshielding of H-2 ′, C-2 ′ and C-4’; shielding of C-3 ′) ( Tables 3 View Table 3 and 4 View Table 4 ), ( Barron et al., 1988). The absence of phenol –OH group signal around δ 13 ppm was consistent with the methoxy group linked to C-5. This was confirmed by the HMBC correlation observed between C-5 and the methoxy protons, the crosspeak observed between the methoxyl and H-6 resonances in the NOESY spectrum, the shielding of the carbonyl (176.1 ppm versus 181.9 ppm for luteolin) and the deshielding of C-3 (106.7 ppm versus 103.1 ppm) due to the absence of the strong internal hydrogen bond with a hydroxyl group at C-5. UV spectra (DAD on-line 266 and 332 nm; UV (MeOH) 266 and 327 nm) are in agreement with the hypsochromic shift of Band I induced by sulfation at position 3’ ( Barron et al., 1988). After acid hydrolysis, compound 3 yielded 5-methoxyluteolin, which was identified by UV and NMR ( Fig. 4 View Fig and S31, Tables 2 View Table 2 and 3 View Table 3 ). All these data allowed identification of 3 as 5-methoxyluteolin 7, 3 ′ -disulfate. Analysis of the 2D NMR data from the COSY, HSQC and HMBC spectra and querying of the SciFinder database confirmed the structure of 3 as an undescribed natural product.
6
Compound 7 eluted at 19.2 min. The positive ESI-MS spectrum gave a quasimolecular peak [M+H] + at 477 m /z, which was compatible with the molecular formula C 16 H 12 O 13 S 2. Another significant ions at m/z 397 [M-80 + H] + and 317 [M-160 + H] + confirmed the presence of two sulfate groups. The 13 C NMR spectrum in DMSO‑ d 6 showed 16 resonances (1 methoxy, 5 CH, and 10 quaternary C including 1 carbonyl). The 1 H NMR spectrum showed a singlet (3H) at δ 3.76 and five proton signals at δ 6.73 (s, H-3), 6.96 (1 H, d, J = 8.6 Hz, H-5 ′), 7.32 (s, H-8), system at 7.71 (1 H, dd, J =2.3, 8.6 Hz, H-6 ′), 7.93 (1 H, d, J =2.3 Hz, H- 2 ′) ( Tables 2 View Table 2 and 3 View Table 3 ). The 1 H and COSY data also indicated the lack of coupling for ring A and the absence of H-6 signal in the aromatic region, suggesting the methoxy group to be attached to C-6. The methoxy position was confirmed by the HMBC correlation between 1 H signal at 3.76 ppm and C-6 at 134.1 ppm. Resonances of carbons and protons showed the typical shifts due to the presence of a sulfate group at position 7 (strong deshielding of H-8 and C-8; shielding of C-7) and 3 ′ (strong deshielding of H-2 ′, C-2 ′ and C-4’; shielding of C-3 ′) ( Barron et al., 1988). UV spectra of compound 7 (DAD on-line 264 and 332 nm; UV (MeOH) 266 and 327 nm) were in agreement with the hypsochromic shift of Band I induced by sulfation at position 3’ ( Barron et al., 1988). After acid hydrolysis, compound 7 yielded nepetin, which was identified by UV, NMR and comparison against an authentic standard (Fig. S31, Tables 2 View Table 2 and 3 View Table 3 ). All these data allowed identification of 7 as nepetin 7, 3 ′ -disulfate. The analysis of the 2D NMR data from the HSQC and HMBC spectra and querying of the SciFinder database confirmed the structure of 7 as an undescribed product.
Compound 14 eluted at 27.4 min. The positive ESI-MS spectrum gave a quasimolecular peak [M+H] + at 397 m /z, which was compatible with the molecular formula C 16 H 12 O 10 S. Another significant ions at m/z 317 [M-80 + H] + and 339 [M-80 +Na] + confirmed the presence of one sulfate group. The 13 C NMR spectrum in DMSO‑ d 6 showed 16 resonances (1 methoxy, 5 CH, and 10 quaternary C including 1 carbonyl) ( Tables 2 View Table 2 and 3 View Table 3 ). The 1 H NMR spectrum showed a singlet (3H) at δ 3.75 and five proton signals at δ 6.15 (s, H-8), 6.44 (s, H-3), 6.93 (1 H, d, J = 8.6 Hz, H-5 ′), 7.58 (1 H, dd, J =2.3, 8.6 Hz, H-6 ′), 7.79 (1 H, d, J =2.3 Hz, H-2 ′). The weak effect observed on H-8 (+0.17) while strong effect is still observed on H-2’ (+0.37) is in agreement with the sulfate group linked at position 3’. The 1 H and COSY spectra also indicated the lack of coupling for ring A and the absence of H-6 signal in the aromatic region, suggesting the methoxy group to be attached to C-6. The methoxy position was confirmed by the HMBC correlation between 1 H signal at 3.75 ppm and C-6 at 133.5 ppm. Resonances of carbon showed the typical shifts due to the presence of a sulfate group at position 3’ (strong deshielding of H-2 ′, C-2 ′ and C-4’; shielding of C-3’ ( Table 4 View Table 4 )). UV spectra of compound 14 (DAD on-line (nm) 237, 273, 335; UV (MeOH) 239, 276, 333) are in agreement with the hypsochromic shift of Band I induced by sulfation at position 3’ ( Barron et al., 1988). After acid hydrolysis, compound 14 yielded nepetin, which was identified by UV, NMR and comparison against an authentic standard (Fig. S31, Tables 2 View Table 2 and 3 View Table 3 ). Altogether, these data identified 14 as nepetin 3 ′ -sulfate. Analysis of HSQC and HMBC spectra confirmed the structure of 14. To our knowledge, this compound has not been reported as a plant natural product before.
Comparison of UV, SM and NMR spectroscopic data of compound 6, 11, 12 and 13 with those reported in the literature allowed their identification as luteolin 7, 3 ′ -disulfate ( 6) ( Barron et al., 1988; Enerstvedt et al., 2017), luteolin 7-sulfate ( 11) ( Barron et al., 1988; Grignon-Dubois and Rezzonico, 2018), nepetin 7-sulfate ( 12) ( Flamini et al., 2001; Tom´as-Barber´an et al., 1987), and luteolin 3 ′ -sulfate ( 13) ( Barron et al., 1988; Enerstvedt et al., 2017; Kim et al., 2016) ( Fig. 3 View Fig and S31, Tables 2–4 View Table 2 View Table 3 View Table 4 , and Supplementary data for details).
2.2.1. Identification of minor flavonoids 2, 4, 8, 9, 15 and 16
Attempts to isolate as pure compound the flavonoids only present in low amounts ( 2, 4, 8, 9, 15, and 16) were unsuccessful. Insufficient sample quantities did not allow further purification and their identity could not be fully confirmed by NMR. However, these minor flavonoids were found structurally related to each other and to the major flavonoids.
Compound 2 was the first flavonoid eluted in the chromatographic run (13.3 min; earlier than all the identified disulfates ( 3, 4, 6, 7). Only present in low amount, it makes it impossible to isolate and it was not detected in the LC/MS spectra. However, some data were available from its chemical behavior. Its HPLC retention time (the lowest observed in the flavonoid series), and on-line UV spectra ( 273, 328 nm) are in agreement with a 6-methoxy flavone substituted by at least two electron - withdrawing groups. Only three 6-methoxy aglycones were obtained after acid hydrolysis of the crude extract, namely nepetin, hispidulin and jaceosidin. Of the three, only nepetin was compatible with retention time of compound 2. The polarity of compound 2, higher than that of nepetin 7, 3 ′ -disulfate could lead to identify it with a trisulfated derivative. We found no mention of nepetin trisulfate in the SciFinder database. UV spectral studies of several naturally occurring and synthetic sulfated compounds indicated that sulfation at ring B induce an important hypsochromic shift in Band I due to the electron withdrawing effect of the sulfate group ( Barron et al., 1988), whereas sulfation at ring A does not influence the UV absorption significantly. In the case of luteolin, Band I UV ( λ max) values were as follows (nm): 7, 3 ′, 4 ′ -trisulfate (305), 7, 4’- (320), 7, 3 ′ -disulfate (333), 4 ′ -sulfate (325), 3 ′ -sulfate (330), 7-sulfate (348) and luteolin (350). Our results with nepetin show the same tendency: 7, 3 ′ -disulfate (330), 3 ′ -sulfate (334), 7-sulfate (348), and nepetin (348). From these values it appears that Band I UV ( λ max) value for compound 2 ( 328 nm) is incompatible with a trisulfate, which led us to tentatively assign compound 2 as nepetin 7, 4 ′ -disulfate. To our knowledge, this product had never been reported.
Compound 8 eluted at 19.6 min. ESI-MS spectrum gave a quasimolecular peak [M+H] + at 381 m /z, which was compatible with the molecular formula C 16 H 12 O 9 S. Another significant ion at m/z 301 [M-80 +H] + confirmed the presence of one sulfate group. UV spectrum (DAD on-line 266 and 341 nm) was in agreement with sulfation at position 7 ( Barron et al., 1988). After acid hydrolysis, compound 8 yielded 5-methoxyluteolin. These results allowed identification of compound 8 as 5-methoxyluteolin 7-sulfate. To our knowledge, this compound has not been reported before.
Comparison of spectroscopic data and chemical behavior of compound 4, 9, 15 and 16 with those reported in the literature allowed their identification as 6-hydroxyluteolin disulfate and monosulfate ( 4 and 9) ( Greenham et al., 2003; Tom´as-Barber´an et al., 1987), hispidulin 7-sulfate ( 15) ( Flamini et al., 2001; Kwak et al., 2016), and jaceosidin 7-sulfate ( 16) (Tom´as-Barber´an et al., 1987; Zhang et al., 2015) (see Supplementary data for details).
2.2.2. Comments on the structural identities as assigned
1 Care should be taken when assigning HPLC peaks 14 and 15 on the only basis of their on-line UV spectra maxima. They present, indeed, very close maxima values ( 14: 273 and 335 nm; 15: 274 and 336 nm). Careful examination of the UV minima of band II and the shape of the absorbance profiles is necessary in this case to achieve a correct assignment as nepetin 3 ′ -sulfate ( 14) and as hispidulin 7-sulfate ( 15). Compound 14 shows a band II λ min at 252 nm with a concave inflexion on both sides, while compound 15 shows a band II λ min at 247 nm with a convex inflexion on both sides (Fig. S21and S31).
2 A purification sub-fraction containing a mixture of disulfate derivatives ( 3, 6, 7) showed a minor compound eluted between 5- methoxyluteolin 7, 3 ′ -disulfate ( 3) and luteolin 7, 3 ′ -disulfate ( 6), which was below the detection level in the crude extract. Its on-line UV spectra (269 and 320 nm) and order of elution are consistent with the presence in the crude extract of traces amounts of luteolin 7, 4 ′ - disulfate ( Barron et al., 1988).
3 Two chromatographic peaks of low intensity and poor resolution were detected in LC/MS. Their spectra showed odd [M+H] + values at 450 and 480, typical of organic substances containing one nitrogen atom, and odd [M-80 + H] + ions at 370 and 400 indicating the presence of one sulfate group, but the low signal-to-noise ratio did not allow exploitation of other fragmentations (Fig. S29 and S30). These data support the presence of traces of flavoalkaloid monosulfates with molecular weight of 449 (Rt ~25 min) and 479 (Rt ~26 min), but their concentration was too low to obtain useable on-line UV spectra. Takagi et al. (1980) had isolated low amounts of a flavoalkaloid derivative from P. iwatensis , which they named phyllospadine (C 21 H 21 NO 6, MW = 383). Its purification required acetylation with acetic anhydride and it was isolated as triacetate of hispidulin 8-(1-methyl-2-pyrrolidinyl) (C 27 H 27 NO 9, MW = 509). Forty mg of phyllospadine tri-acetate were obtained from two kg of dried plant, accounting for 15 μg gdw 1 of phyllospadine. Takagi et al. (1979) also analyzed the flavonoid content of P. japonicus , but phyllospadine was not found in this species. Neither phyllospadine nor its hypothetical sulfate was found in our extracts, but the two nitrogeneous compounds detected in the present study would probably share a number of similarities with this substance. Flavonoid alkaloids are of interest due to their rarity, their amphoteric nature, and the biological activity of some of their natural sources. They result from the convergence of two distinct biosynthetic pathways, affording natural products with a wide range of interesting biological activities that would not be expected for flavonoids or alkaloids alone ( Blair et al., 2017). Our next step in this respect will be to collect the large amounts of plant material needed to isolate and characterize these very minor compounds.
2.3. Inter-annual variability in the phenolic content of P. torreyi View in CoL View at ENA
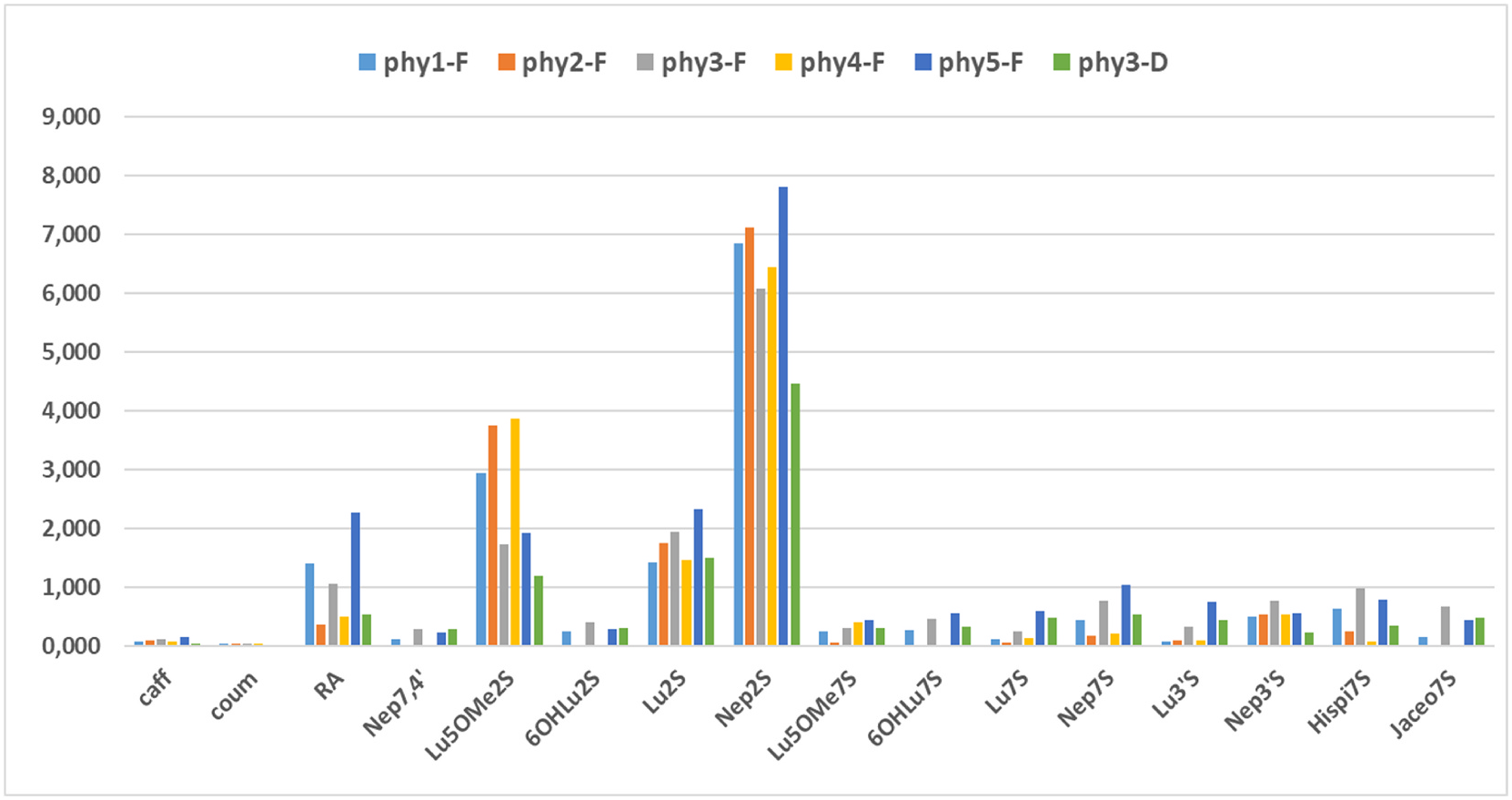
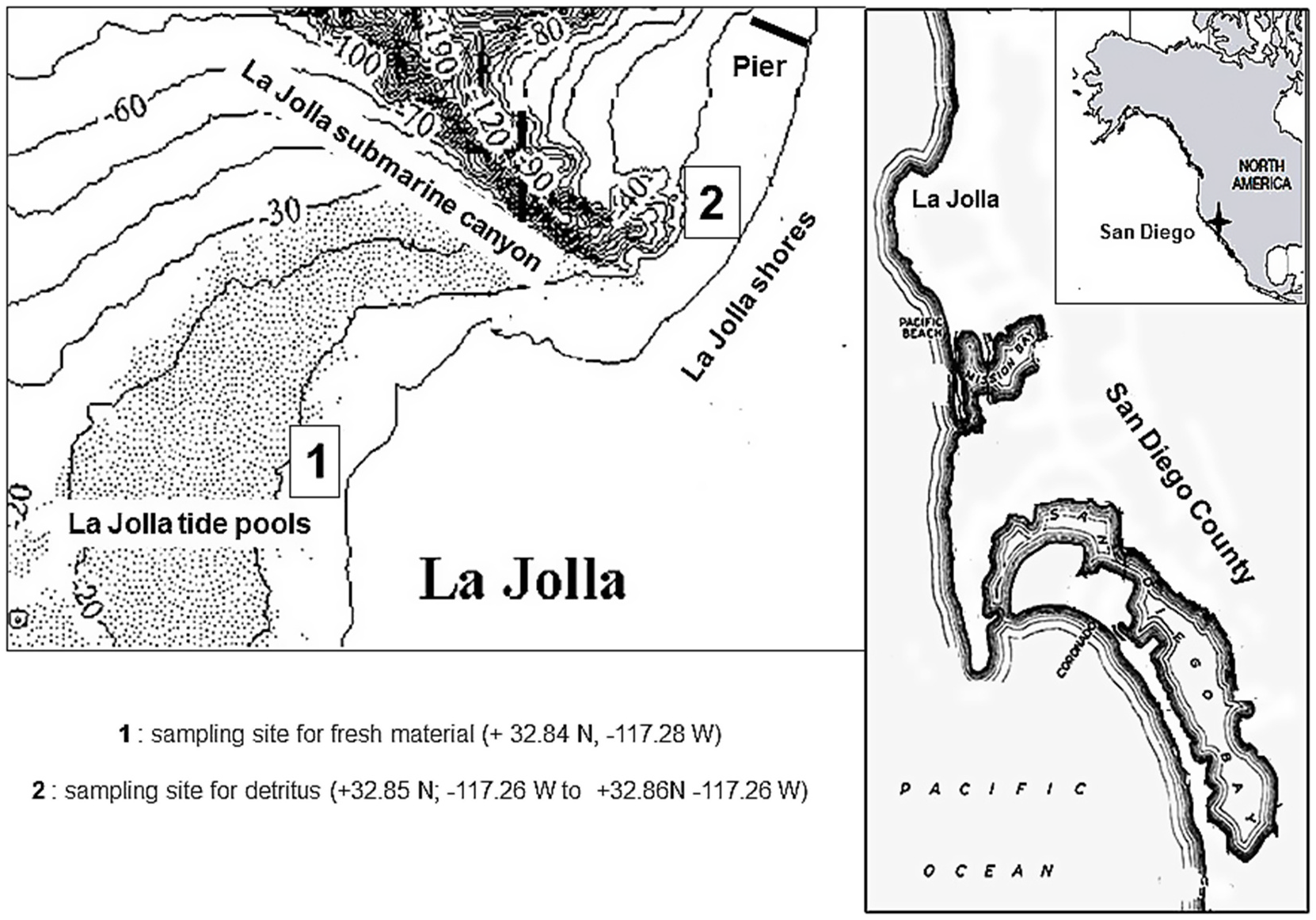
A total of five sampling campaigns of fresh samples of the aerial part of P. torreyi (batch number: PhyF 1–5, each constituted of three independent samples) were conducted in La Jolla ( Fig. 1 View Fig ) over different years (2015–2017) and seasons (summer and winter). Aqueous-methanolic extracts were prepared from each collection and individual phenolic compounds were quantified by HPLC in each of the fifteen crude extracts. The quantitative variation of flavonoids and phenolic acids was found to be relatively consistent from year to year. Comparison of the summer extracts showed similar HPLC profile and a weak variability in the phenolic concentrations. For all samples, the flavonoid pattern was found largely dominated by the disulfates ( 3, 6 and 7). Nepetin 7, 3 ′ - disulfate ( 7) had the highest content among all compounds in all tested samples ( 6.07–7.81 mg g 1) and represented 41–52% of the flavonoid pool. The second most abundant product was 5-methoxyluteolin 7, 3 ′ - disulfate ( 3, 1.73–3.86 mg g 1; 12–29%), followed by luteolin 7, 3 ′ - disulfate ( 6; 1.42–2.32 mg g 1; 10–13%). The minor flavonoids 2, 4 and 9 were only detected in the summer samples. The flavonoid 7-sulfates ( 8, 11, 12, and 15) and 3 ′ -sulfates ( 13, 14) were present in all the samples in moderate amounts, while jaceosidin 7-sulfate ( 16) was detected only in summer. Low amounts of caffeic- ( 1) and coumaric acid ( 5) were found in all the samples ( 0.075 –0.158 mg g 1 and 0.027 –0.041 mg g 1 respectively). These two phenolic acids are very common in seagrasses ( Zapata and McMillan, 1979). Seasonality was observed for rosmarinic acid ( 10) whose concentration was higher in summer ( 1.057 –2.264 mg g 1) than in winter ( 0.361 –0.491 mg g 1). We had previously isolated rosmarinic acid from Z. marina and Z. noltei, which also belong to the Zosteraceae family ( Achamlale et al., 2009). This is the first report of rosmarinic acid in a member of the genus Phyllospadix . The results are summarized in Table 1 and Fig. 4 View Fig , which show the average concentrations of the three harvest replicates for each sampling campaign.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |