Phasmotaenia Navás, 1907
|
publication ID |
https://doi.org/ 10.5281/zenodo.185796 |
|
DOI |
https://doi.org/10.5281/zenodo.6224518 |
|
persistent identifier |
https://treatment.plazi.org/id/9F7B87E1-436B-FF8C-E2AE-633AFE2BF87D |
|
treatment provided by |
Plazi |
|
scientific name |
Phasmotaenia Navás, 1907 |
| status |
|
Phasmotaenia Navás, 1907 View in CoL
Type-species: Taeniosoma sanchezi Bolívar, 1897: 31 , by indication.
Phasmotaenia Navás, 1907: 10 View in CoL . [Replacement name for preoccupied Taenionema Bolívar, 1906 View in CoL ] Vanschuytbroeck & Cools, 1981: 17.
Zompro & Eusebio, 2000: 61.
Huang & Brock, 2001: 9.
Bragg, 2001: 641.
Eusebio et al., 2004: 191.
Zompro, 2004: 319.
Otte & Brock, 2005: 266.
Bresseel & Hennemann, 2008: 18.
Chen & He, 2008: 345, 454.
Hennemann & Conle, 2008: 57.
Phasmotaenionema, Karny, 1923: 240 View in CoL . [Misspelling of Phasmotaenia Navás, 1907 View in CoL ] Günther, 1933: 155
Günther, 1937: 8.
Günther, 1953: 555.
Phasmatotaenionema, Bradley & Galil, 1977: 193. [Misspelling of Phasmotaenia Navás, 1907 ]
Taeniosoma Bolívar, 1897: 30 View in CoL . Type-species: Taeniosoma sanchezi Bolívar, 1897: 31 , by monotypy. [Preoccupied by Taeniosoma Stimpson, 1857: 162 (Nematoda) ]
Kirby, 1904: 361.
Redtenbacher, 1908: 442.
Bruner, 1915: 41.
Taenionema Bolívar, 1906: 393 View in CoL . Type-species: Taeniosoma sanchezi Bolívar, 1897: 31 , by indication. [Replacement name for preoccupied Taeniosoma Bolivar, 1897 . Preoccupied by Taenionema Banks, 1905: 56 View in CoL , ( Plecoptera View in CoL ) and replaced by Phasmotaenia Navás, 1907 View in CoL ]
Kirby, 1910: 569.
Taeniophasma Uvarov, 1940: 379 View in CoL . Type-species: Taeniosoma sanchezi Bolívar, 1897: 31 , by indication. [Unnecessary replacement name for the preoccupied Taenionema Bolívar, 1906 View in CoL , already replaced by Phasmotaenia Navás, 1907 View in CoL ]
Hermarchus, Redtenbacher, 1908: 444 View in CoL , pl. 21: 5, 5a (not Stål, 1875 – in part). Günther, 1937: 8.
Otte & Brock, 2005: 155 (in part).
Stephanacris, Günther, 1936: 339 View in CoL , fig. 14.
Hennemann & Conle, 2006: 19, figs. 9–11, 16, 21 (in part).
Description: Ƥ, 33: Moderately sized to large (body lengths: 3 81.6–117.0 mm, ƤƤ including subgenital plate 129.5–189.0 mm) and very slender to moderately robust representatives of the tribe. Head longer than wide-sub-oval to globose with the vertex ranging from almost flat to moderately convex; unarmed. Eyes circular and projecting hemispherically. Antennae consisting of 25–32 segments and ± reaching to posterior margin of metanotum (3), or at least reaching half way along the mesonotum (ƤƤ). The basal segments shortened, those in the median portion elongate and gradually shortening towards apex of antennae. Pronotum narrower than head. Mesothorax ± distinctly swollen pre-medially in ƤƤ, slender in 3
and at least 3.5x longer than pronotum. Mesonotum either smooth, slightly rugulose, granulose or armed with a cluster of spines in anterior portion. Mesosternum smooth or sparingly tuberculose or spinose (ƤƤ only); metasternum unarmed. Meso- and metapleurae of ƤƤ unarmed or with a longitudinal row of tubercles or moderately sized spines. Metanotum rectangular. Tegmina ranging from very small and vestigial to scale-like; at best a little longer than metanotum. Size of alae in ƤƤ ranging from very small and vestigial to roughly equal in length to the median segment; anal region red, black, or red with a black outer margin. In 3 alae either very small and vestigial, or well developed and projecting as far as to abdominal segment VI; anal region transparent. Median segment either slightly shorter (apterous 3) or longer than the metanotum. Abdomen excluding median segment longer than head and complete thorax combined. Segments II–VII longer than wide and ± parallel-sided; occasionally with a posterolateral lobe. Tergites II–VII either smooth or more rarely minutely wrinkled; V and VI in ƤƤ occasionally with a scale-like posteromedian hump. Praeopercular organ on sternum VII indistinct and usually formed by a small posteromedian tubercle or granule. Tergites VIII–X in ƤƤ narrower and considerably shorter than previous, X with a longitudinal median carina and a shallow, ± triangular median excavation or indention. Supraanal plate small, roundly triangular and slightly projecting underneath anal segment (= tergite X). In 3 tergites VIII–X roughly equal in width to previous segments, X with a shallow median excavation. Outer angles of anal segment (= tergite X) interiorly armed with a variable number small, of in-curving spines. Vomer well developed and sclerotised, roughly triangular and with a single hook-like terminal point. Cerci of both sexes small, subcylindrical and narrowed towards the apex in ƤƤ. Poculum of 3 moderately bulgy, convex and with a ± distinct, blunt central hump; reaching about half way along anal segment. Subgenital plate of ƤƤ longitudinally keeled and projecting over apex of abdomen by at least the length of anal segment, occasionally strongly elongated, lanceolate and projecting beyond abdomen by almost twice the length of tergites VIII–X combined. Upper gonapophyses elongated and slightly projecting under anal segment, the lower gonapophyses very long, filiform and ± reaching to tip of subgenital plate; apex gently up-curving. Profemora compressed and curved basally, slightly triangular in cross-section with the anterodorsal carina ± raised and longer than mesothorax. All carinae, except anteroventral carina, ± distinctly dentate; medioventral carina set with a variable number of small spines. Occasionally a small, dentate sub-apical lobe is present on the posteroventral carina. Protibiae slender and sparsely serrate or dentate ventrally. Meso- and metafemora trapezoidal in cross-section with the dorsal carinae nearing. Outer ventral carinae ± prominently dentate, the dorsal carinae either smooth or very sparsely dentate. In ƤƤ occassionally a ± distinct, dentate sub-apical lobe is present on one or both of the outer ventral carinae; in one case also the dorsal carinae bear an enlarged sub-apical tooth. Medioventral carina distinct and set with a variable number of moderately sized spines. Meso- and metatibiae slender and trapezoidal in cross-section with all carinae dentate to a variable degree, dorsal carinae may be smooth and the posterodorsal carina may be gently elevated sub-basally. All tarsi slender in 3, the basitarsus longer than the following three tarsomeres combined. In ƤƤ the probasitarsus often has the dorsal carina ± distinctly raised and rounded. Ventral carinae of all basitarsi in ƤƤ minutely dentate.
Eggs: Small to medium-sized (overall length 3.6–4.3 mm), capsule ovoid, round to slightly oval in crosssection and at least 1.5x longer than wide. Dorsal surface more strongly convex than ventral surface. Entire capsule surface smooth and strongly shiny. Colouration ranging from almost plain dark brown or black to pale cream or grey furnished with darker brown or blackish markings. Micropylar plate rhomboidal or spearshaped with the lower portion ± narrowed; usually> 1/3 the length of capsule. Micropylar cup placed near to centre of micropylar plate, the internal plate closed. Operculum circular to slightly oval, in the centre elevated into an irregularly shaped, knob-like or conical pseudo-capitulum.
Variation: Several features of the adult ƤƤ in particular are seen to underlie considerable intraspecific variability. This particularly concerns to the length of the tegmina and alae (e.g. P. australe (Günther, 1933)) , armature of the legs (e.g. P. salomonense n. sp.), and colouration (almost all species). Several of these variations appear to be specific for distinct island colonies, but any broader discussion or affiliation of certain variations to definite islands, clearly deserve more material from precise localities. Although the degree of the pre-medial swelling of the mesothorax of ƤƤ may at first glance appear a useful feature for the distinction of species, captive breeding of P. australe ( Günther, 1933) , P. lanyuhensis ( Huang & Brock, 2001) and P. spinosa n. sp. has shown this to depend on whether the insects have or have not been feeding recently. The mesothorax swells remarkably during the feeding process at the night and may double its diameter. During the day when the food is digested, the diameter and swelling decrease again with the mesothorax becoming considerably more slender.
Comments: The valid name of this genus has over decades been confused by former authors. Bolívar (1897) first described Taeniosoma upon a single species, T. sanchezi , from the Philippines. Since this generic name was already in use for a genus of the Nematoda, Bolívar (1906: 393) subsequently introduced Taenionema as a replacement name. But Ta enion ema as well was a preoccupied name and replaced by Phasmotaenia Navás, 1907 . Obviously unaware of the latter publication, Uvarov (1940: 379) unnecessarily introduced Taeniophasma as a replacement name for the already replaced Taenionema Bolívar, 1906 . Günther (1933, 1937 and 1956) misspelled the genus “ Phasmotaenionema ” and Bradley & Galil (1977: 193) misspelled it “ Phasmatotaenionema ”, but both authors correctly referred to Navás (1907) as the author. A clarification was provided by Vanschuytbroeck & Cools (1981: 17).
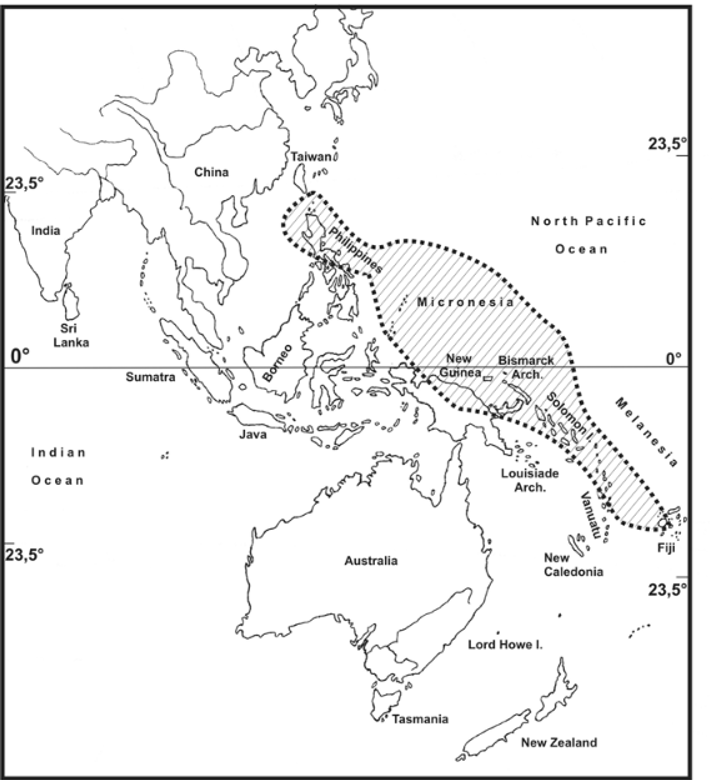
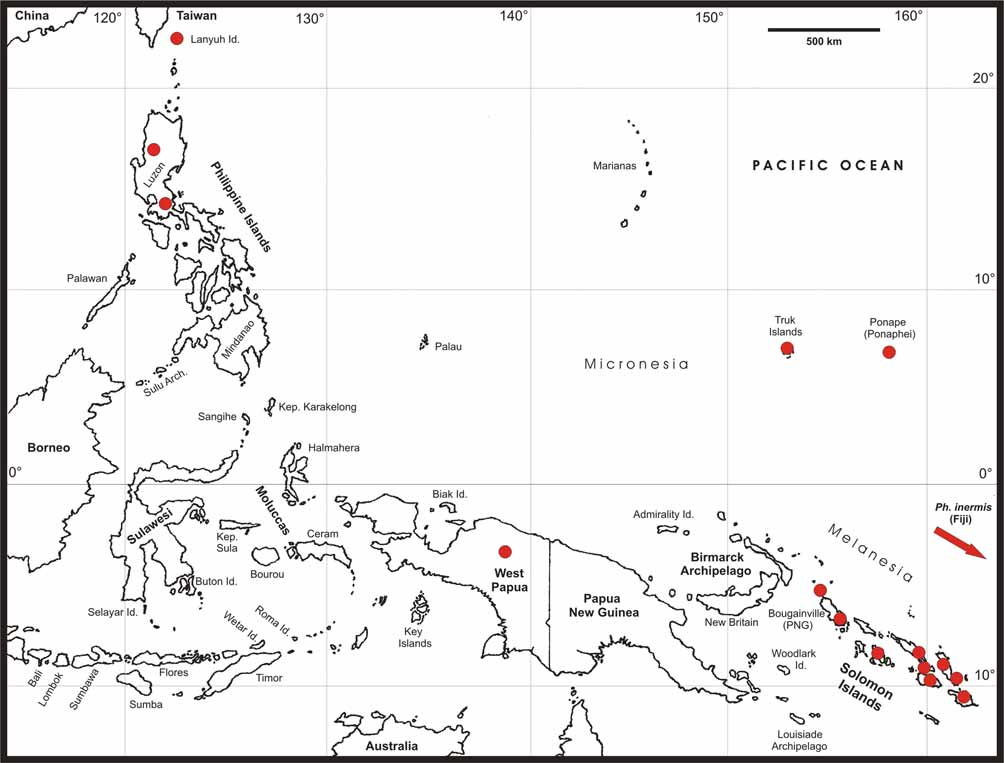
Günther (1933) added to the genus P. australe , a species from the Solomon Islands, but subsequently ( Günther, 1937: 8) decided it was a synonym of Hermarchus godeffroyi Redtenbacher, 1908 from Micronesia and better placed in the genus Hermarchus Stål, 1875 . As shown herein neither act of Günther (1937) is correct, P. australe ( Günther, 1933) being a valid species and a member of Phasmotaenia Navás, 1907 . A third species, Phasmotaenia lanyuhensis Huang & Brock, 2001 , was recently described from Lanyuh Island south of Taiwan. Due to Günther’s misinterpretation of P. australe , the genus Phasmotaenia was currently believed to be restricted to the northern Philippine Islands and Lanyuh Island ( Huang & Brock, 2001; Eusebio, Lit & Vörkel, 2004). Its distribution is however here shown to extend eastward to Micronesia, the Solomon Islands, New Guinea and Fiji (see distribution below, Fig. 1 View FIGURE 1 ).
Phasmotaenia Navás, 1907 View in CoL is remarkable for containing certain species in which the wings are better developed in ƤƤ than in corresponding 3 ( P. sanchezi ( Bolívar, 1897) and P. lanyuhensis Huang & Brock, 2001 View in CoL ). In both species the tegmina and alae are very small and vestigial (<1.5 mm) in 3 whereas at least the alae are roughly half as long as the median segment (length> 5.0 mm) and exhibit a small, but developed black anal fan. Normally, in the Phasmatodea View in CoL 3 exhibit better developed wings than corresponding ƤƤ.
A few remarks about the habits and remarkable defensive reactions of the insects may be of interest. Nymphs and adults frequently react very excited when disturbed and brachypterous ƤƤ (e.g. P. s p i n o s a n. sp., Fig. 63 View FIGURES 62 – 64 ) usually display their colourful hind wings. This may include curling up the abdomen and pinching at the predator with the hind legs. Mating usually takes place at night and lasts for 2–3 hours, the 3 using their hook-like vomer to hold fast to the ƤƤ praeopercular organ on sternum VII. Due to the relatively small size of the eggs, if compared to the adult insects, ƤƤ are quite prolific egg-layers each producing up to 15 eggs per day and several hundreds in their entire live. The eggs are flicked away singularly by an abrupt movement of the abdomen. Winged 3 are capable of active flight and may also flash their wings when disturbed.
For a differentiation of Phasmotaenia from related genera see Table 1 below.
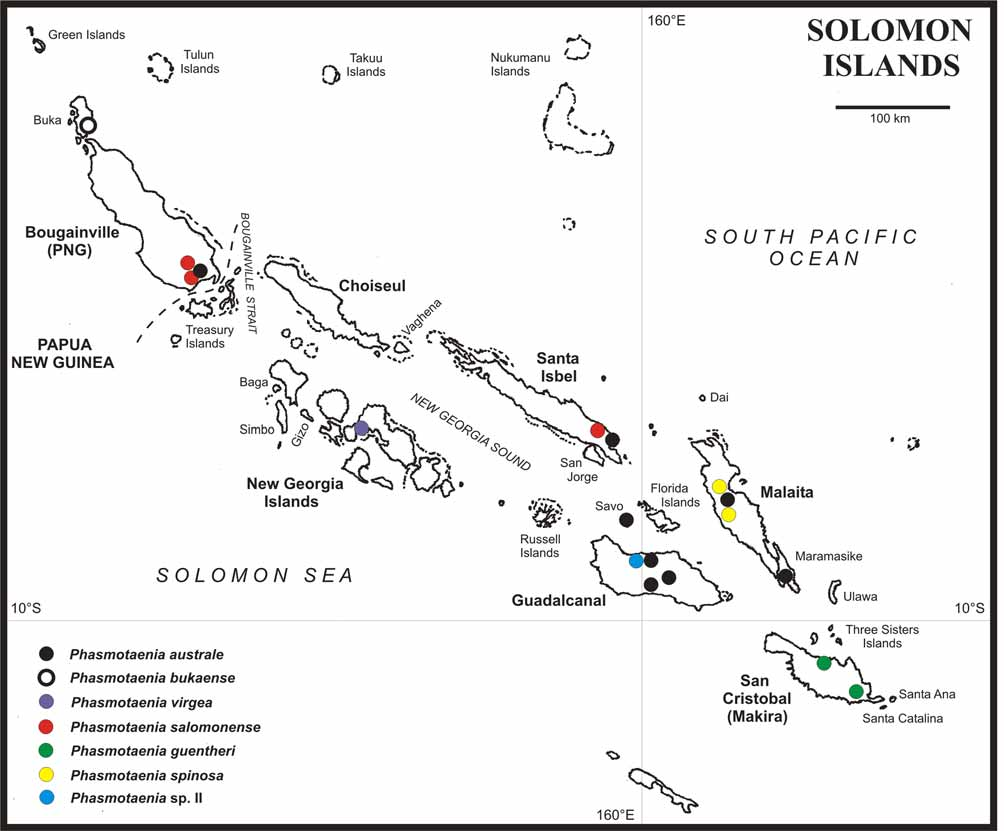
Distribution ( Figs. 1 View FIGURE 1 , 59 View FIGURE 59 & 60 View FIGURE 60 ): Philippines (Luzon Id.), Lanyuh Island SE of Taiwan, Micronesia ( Ponape & Truk), Solomon Islands, New Guinea (Mount Doorman) and Fiji ( Viti Levu). So far, no members have been recorded from the intervening islands e.g. Moluccas, Palau, the Bismarck Archipelago, New Hebrides or Tuvalu, which may be explained by the generally poor degree of exploration of the phasmtodean fauna of these regions.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
InfraOrder |
Anareolatae |
|
Family |
|
|
Tribe |
Stephanacridini |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
InfraOrder |
Anareolatae |
|
Family |
|
|
Tribe |
Stephanacridini |
|
Genus |
Phasmotaenia Navás, 1907
| Hennemann, Frank H. & Conle, Oskar V. 2009 |
Taeniophasma
| Uvarov 1940: 379 |
| Bolivar 1897: 31 |
Stephanacris, Günther, 1936 : 339
| Gunther 1936: 339 |
Phasmotaenionema
| Gunther 1933: 155 |
| Karny 1923: 240 |
Hermarchus
| Gunther 1937: 8 |
| Redtenbacher 1908: 444 |
Phasmotaenia Navás, 1907 : 10
| Vanschuytbroeck 1981: 17 |
| Navas 1907: 10 |
Taenionema Bolívar, 1906 : 393
| Bolivar 1906: 393 |
| Bolivar 1897: 31 |
Taeniosoma Bolívar, 1897 : 30
| Bolivar 1897: 30 |
| Bolivar 1897: 31 |