Amphisbetia distans ( Lamouroux, 1816 )
|
publication ID |
https://doi.org/10.11646/zootaxa.4689.1.1 |
|
persistent identifier |
https://treatment.plazi.org/id/9E4CE23A-FF81-F10E-FF03-60DBFA7429F1 |
|
treatment provided by |
Plazi |
|
scientific name |
Amphisbetia distans ( Lamouroux, 1816 ) |
| status |
|
Amphisbetia distans ( Lamouroux, 1816) View in CoL
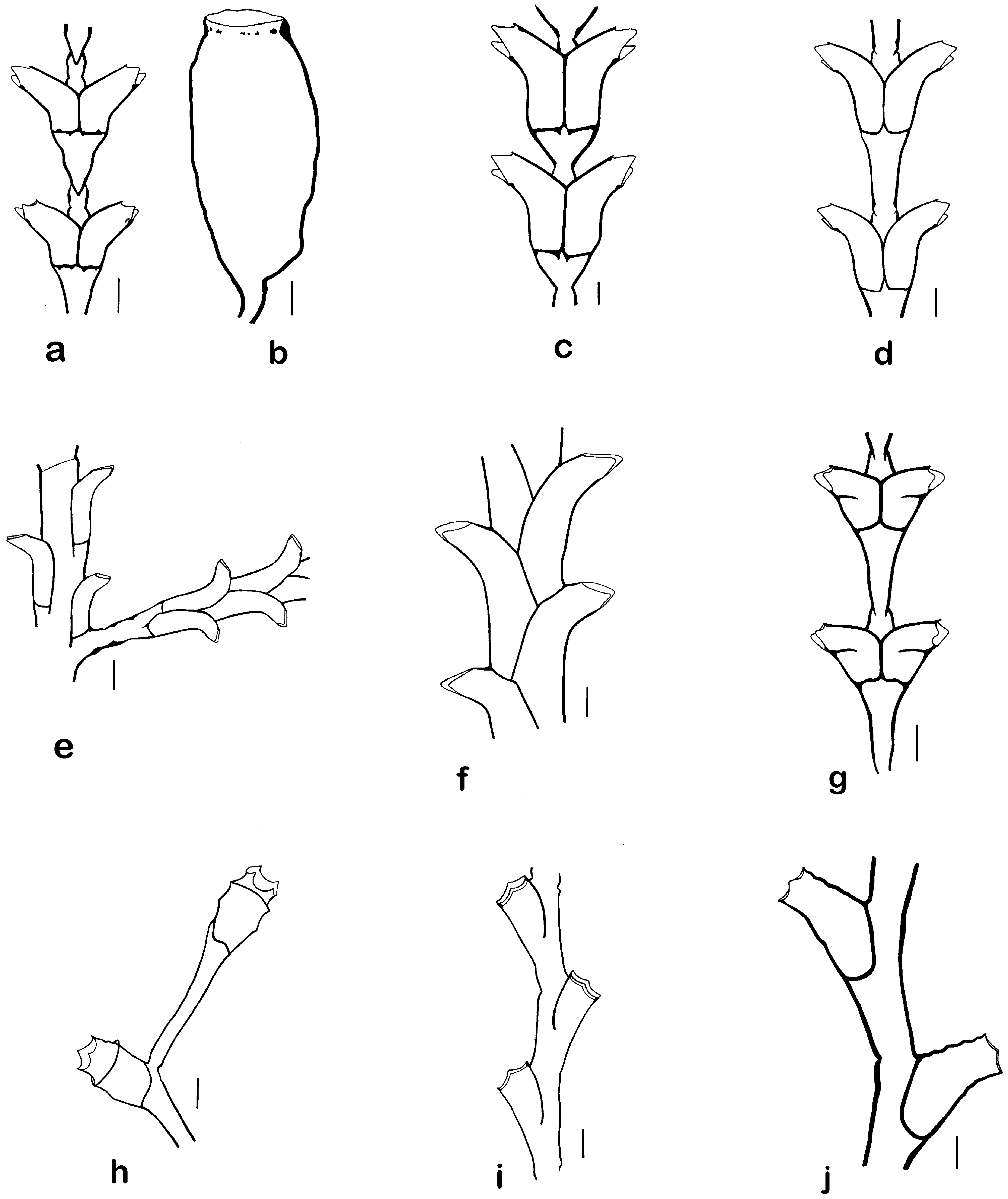
Figs. 21a, b View FIGURE 21
Dynamena distans Lamouroux, 1816: 180 View in CoL , pl. 5, figs. 1a, B.
Sertularia stookeyi View in CoL .— Joyce, 1961: 66, pl. 16, figs. 3, 4.— Shier, 1965: 55, pls. 27, 30.
Type locality. Atlantic Ocean : “ Sur le Fucus natans ( Sargassum natans ) et quelques autres productions marines…” ( Lamouroux 1816: 180) .
Material examined. Sanibel Island, beach at Lighthouse Point, 26°26’57”N, 82°01’06”W, on detached Syringodium at water’s edge, 13 March 2018, 20° C, 33.5‰, one colony, up to 7 mm high, with gonophores, coll. D. Calder, ROMIZ B4386.—Sanibel Island, beach at Lighthouse Point, 26°26’58”N, 82°01’04.5”W, on detached Thalassia at water’s edge, 21 March 2018, 22° C, 34.5‰, two colonies, up to 5 mm high, without gonophores, coll. D. Calder, ROMIZ B4387.—Sanibel Island, beach at Lighthouse Point, 26°26’38”N, 82°01’36”W, on stranded Thalassia and Syringodium , 28 March 2018, 21° C, 35‰, three colonies, up to 7 mm high, without gonophores, coll. D. Calder, ROMIZ B4388.
Remarks. The hydroid described by Lamouroux (1816) as Dynamena distans is now widely known as either Sertularia distans or Tridentata distans . As noted in previous works ( Calder 2013; Calder et al. 2019), molecular phylogenetic studies ( Moura et al. 2011; Maronna et al. 2016) reveal that the species is more closely related to Sertularia operculata Linnaeus, 1758 , type species of Amphisbetia L. Agassiz, 1862 , than to either Sertularia argentea Linnaeus, 1758 , type species of Sertularia Linnaeus, 1758 or Sertularia perpusilla Stechow, 1919 , type species of Tridentata Stechow, 1920 . The binomen Amphisbetia distans has therefore been applied to it here, following Calder et al. (2019). Sertularia stookeyi Nutting, 1904 is a subjective junior synonym ( Calder 1983, 1990 [1991a]).
Records below indicate that A. distans is widely distributed in shallow tropical and temperate waters of the western North Atlantic. It has been reported from many locations in the Caribbean Sea, and its known range extends as far north as southern Massachusetts on the east coast of the United States ( Fraser 1944). A eurytopic species ( Calder 1976, 1990), it is particularly abundant and widespread in estuaries of South Carolina ( Calder 1983). The hydroid was also found all seasons of the year on the shallow continental shelf nearby ( Wenner et al. 1984). By contrast, records so far from the Gulf of Mexico are relatively few. While a substrate generalist, the hydroid is well-known as an epibiont on pelagic Sargassum . As such, it occurs in surface waters of the Sargasso Sea, and is transported northwards on gulfweed in the Gulf Stream. Elsewhere, the range of the species extends southwards to Brazil in the western Atlantic ( Oliveira et al. 2016), and it is reported to be circumglobal in tropical and temperate waters ( Calder 2013). While the hydroid of A. distans has been identified from bottoms as deep as 826 m ( Ramil & Vervoort 1992), it is predominantly a species of shallow waters (< 60 m) ( Cornelius 1995b). Amphisbetia distans has also been found in the intertidal zone ( Calder 2013).
Detailed taxonomic accounts of this species include those of Calder (1990 [1991a], 2013, as Tridentata distans ), Cornelius (1995b, as T. distans ), Medel and Vervoort (1998, as Sertularia distans ), and Calder et al. (2019).
Reported Distribution. Gulf coast of Florida. Seahorse Key, on Thalassia and Syringodium ( Joyce 1961: 66, as Sertularia stookeyi ).— Cape San Blas area ( Shier 1965: 55, as Sertularia stookeyi ).
Elsewhere in western North Atlantic. USA: Massachusetts, off Hyannis, on Sargassum ( Verrill 1875b: 43, as Sertularia gracilis ).— USA: Massachusetts, Naushon Island ( Nutting 1904: 57, as Sertularia gracilis ).— Bahamas: Great Bahama Bank, on floating seaweed ( Nutting 1904: 60, as Sertularia stookeyi ).— USA: Massachusetts, Vineyard Sound, Naushon Island, outside Tarpaulin Cove, 7–8 ftm ( 13–15 m), on Fucus , Thuiaria argentea (= Sertularia argentea ) ( Fraser 1912a: 47, as Sertularia stookeyi ).— USA: North Carolina, Beaufort area, on floating gulfweed and seaweed + Bogue Sound, 10 ft ( 3 m) + North River, 10 ft ( 3 m) + Straits, 10 ft ( 3 m) + offshore, on sponge ( Fraser 1912b: 375, as Sertularia stookeyi ).— Bermuda: off north shore, on floating Sargassum + Hamilton Harbour, on floating Sargassum ( Bennitt 1922: 251, as Sertularia stookeyi ).— Bermuda: on floating Sargassum ( Prat 1935: 127, as Sertularia gracilis ; 1940: 272, as Sertularia gracilis ).— Bonaire: Plaja Oranje Pan, on stranded algae + Boca Onima, on stranded Sargassum (Leloup 1935: 48, as Sertularia distans var. gracilis ).— Bonaire: Lac, mouth, back of reef, 1.5 m, on detached Sargassum (Leloup 1935: 48, as Sertularia distans var. gracilis forme peculiaris).— Curaçao: Boca Grandi, on stranded Sargassum + Boca Grandi, on deteriorated, floating Sargassum (Leloup 1935: 48, as Sertularia distans var. gracilis ).— Aruba: Boca Prins, on stranded Sargassum (Leloup 1935: 48, as Sertularia distans var. gracilis ).—Sargasso Sea: 39°N, 41°W, W of the Azores, on floating Sargassum (Leloup 1935: 48, as Sertularia distans var. gracilis ).—Sargasso Sea: 32°07’N, 66°35’W, W of Bermuda, on floating Sargassum ( Leloup 1937: 105, as Sertularia distans var. gracilis ).—Gulf Stream and Sargasso Sea: on Sargassum natans and S. fluitans (Burkenroad, in Parr 1939: 23, as Sertularia flowersi ). — USA: Florida, between Biscayne and Duck keys ( Fraser 1943: 93, as Sertularia stookeyi ).— Trinidad & Tobago: Trinidad, Maguaripe Bay (=Macqueripe Bay) ( Fraser 1943: 93, Sertularia stookeyi ).— USA: Massachusetts, Nantucket Sound, 18 ftm ( 33 m) + Vineyard Sound, near West Chop Light, 14 ftm ( 26 m) + Vineyard Sound, Naushon Island, off Tarpaulin Cove, 14 ftm ( 26 m) ( Fraser 1944: 289, as Sertularia stookeyi ).—Gulf Stream: S of Marthas Vineyard (Massachusetts), 39°56’N, 70°46’W (probably on Sargassum ) ( Fraser 1944: 289, as Sertularia stookeyi ).— Panama: Caledonia Bay (Puerto Escoces), on floating Sargassum ( Fraser 1947b: 11, as Sertularia stookeyi ).— Venezuela: Isla Cubagua, shore ( Fraser 1947b: 11, as Sertularia stookeyi ).— Aruba: Boca Prins, on stranded Sargassum ( Van Gemerden-Hoogeveen 1965: 36, as Sertularia distans var. gracilis ).— Curaçao: Boca Grandi, on stranded Sargassum ( Van Gemerden-Hoogeveen 1965: 36, as Sertularia distans var. gracilis ).— Bonaire: Kralendijk, Pasanggrahan, on wood fragments + Oranjepan, on stranded Sargassum + Boca Washikemba, on stranded brown algae ( Van Gemerden-Hoogeveen 1965: 36, as Sertularia distans var. gracilis ).— Trinidad & Tobago: Tobago, Rockley Bay (=Rockly Bay), on Sargassum ( Van Gemerden-Hoogeveen 1965: 36, as Sertularia distans var. gracilis ).— Venezuela: Islote Aves, northern lagoon, near low water ( Van Gemerden-Hoogeveen 1965: 36, as Sertularia distans var. gracilis ).— Antigua and Barbuda: Antigua, Deep Bay at Fort Barrington, on Sargassum ( Van Gemerden-Hoogeveen 1965: 36, as Sertularia distans var. gracilis ).— Antigua and Barbuda: Barbuda, Martello Tower Beach, near low tide ( Van Gemerden-Hoogeveen 1965: 36, as Sertularia distans var. gracilis ).— St. Kitts and Nevis: St. Kitts, Frigate Bay, near low tide, on Sargassum (Van Gemerden- Hoogeveen 1965: 36, as Sertularia distans var. gracilis ).— Saint-Barthélemy: Public, tidal zone, on Sargassum ( Van Gemerden-Hoogeveen 1965: 36, as Sertularia distans var. gracilis ).— Bahamas: North Bimini, 1 km offshore ( Van Gemerden-Hoogeveen 1965: 36, as Sertularia distans var. gracilis ).—Sargasso Sea: 43°04’N, 31°W, N of the Azores ( Van Gemerden-Hoogeveen 1965: 36, as Sertularia distans var. gracilis ).—Sargasso Sea + Gulf Stream, stations between Florida and South Carolina, on Sargassum polyceratium , S. filipendula ( Rackley 1974: 39, as Sertularia stookeyi ).— USA: South Carolina, inshore waters, abundant, especially on Leptogorgia ( Calder & Hester 1978: 91, as Sertularia stookeyi ).— Belize: Carrie Bow Cay, on Thalassia ( Spracklin 1982: 246, as Sertularia stookeyi ).— USA: South Carolina estuaries, Bulls Bay, 4–5 m + Sewee Bay, 2–4 m + Prices Creek, 8 m + Inlet Creek, 4 m + Charleston Harbor, entrance, 10 m + Charleston Harbor, Rebellion Reach, 12 m + Stono Inlet, 7–10 m + Kiawah River, 6 m + Dawho River, 7–10 m + North Edisto River, Bear’s Bluff, 7 m + North Edisto River, Toogoodoo Creek, 4 m + North Edisto River, Steamboat Creek + North Edisto River, Wadmalaw Island, 8 m + North Edisto River, Point of Pines, 8 m + North Edisto River, Deveaux Bank, 10 m + South Edisto River, Bay Point + Beaufort River, 6 m + Colleton River, 6 m + Port Royal Sound, 8 m + Calibogue Sound, 7 m ( Calder 1983: 13, as Sertularia distans ).— USA: South Carolina, inner ( 17–18 m) and middle ( 32–36 m) continental shelf + Georgia, inner ( 17–22 m), middle ( 23–29 m) and outer ( 59–67 m) continental shelf ( Wenner et al. 1984: 21, 40, as Sertularia distans ).— USA: Texas, mid-continental shelf ( Rezak et al. 1985: 224, as Sertularia distans ).— USA: South Carolina, coastal areas, in stomachs of Atlantic spadefish ( Hayse 1990: 81, as Sertularia distans ).— Bermuda: Harrington Sound, Stream Passage Cave, inside entrance, 0.5 m, on rock + Castle Harbour, near Castle Island, on Thyroscyphus marginatus , 5 m + Harrington Sound, Cripplegate Cave, entrance, 0.5 m, on rock + Flatts Inlet, on algae, 2–3 m + Natural Arches Beach, on stranded Sargassum (Calder 1990 [1991a]: 105, 106, as Tridentata distans ).— Belize: Twin Cays, on Rhizophora , Thalassia , other invertebrates ( Calder 1991b: 223, 1991c: 2068, as Tridentata distans ).— Bermuda: Harrington Sound, just below tidal level ( Thomas 1996: 758, as Sertularia distans ).— Cuba: Golfo de Batabanó, Cayo Real, 21°57’42.5”N, 83°32’06.2”W, 0 m + Cayo Los Indios, 22°02’28.9”N, 82°50’55.4”W, 0.5 m (Castellanos et al. 2011: 22, as Tridentata distans ).— Cuba: Isla de la Juventud, wreck of Las Calderas, 21°29’57.5”N, 82°38’57.3”W, 6 m (Castellanos et al. 2011: 22, as Tridentata distans ).— USA: Florida, Fort Pierce Inlet, north jetty, on Thyroscyphus ramosus , intertidal ( Calder 2013: 30, as Tridentata distans ).—French Lesser Antilles, Martinique: Le Vauclin, Pointe Faula, 14.54064, -60.82837, 0 m, on floating Sargassum ( Galea & Ferry 2015: 234, as Sertularia distans ).— Mexico: Alacranes Reef, on shipwreck ( Mendoza-Becerril et al. 2018b: 130, as Sertularia distans ).— Cuba: Havana, coral reef system west of the city (Castellanos et al. 2018: Supplementary Table S2, as Sertularia distans ).— Panama: Bocas del Toro area, near Bocatorito Bay ( Miglietta et al. 2018b: 108, as Sertularia distans ).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
SubClass |
Hydroidolina |
|
Order |
|
|
Family |
|
|
Genus |
Amphisbetia distans ( Lamouroux, 1816 )
| Calder, Dale R. 2019 |
Sertularia stookeyi
| Shier, C. F. 1965: 55 |
| Joyce, E. A. Jr. 1961: 66 |
Dynamena distans
| Lamouroux, J. V. F. 1816: 180 |