Olea hensoni, Filho & Paulay & Krug, 2019
|
publication ID |
https://doi.org/10.11646/zootaxa.4614.3.7 |
|
publication LSID |
lsid:zoobank.org:pub:79166C20-0D37-49B5-B08B-CBEE10B392C3 |
|
persistent identifier |
https://treatment.plazi.org/id/9E3D87DC-7F1C-FFFA-A6F9-E19FFC827DA0 |
|
treatment provided by |
Plazi |
|
scientific name |
Olea hensoni |
| status |
|
Genus Olea Agersborg, 1923 View in CoL
Olea hensoni new species
( Figures 1 View FIGURE 1 , 4–7 View FIGURE 4 View FIGURE 5 View FIGURE 6 View FIGURE 7 )
Zoobank registration: urn:lsid:zoobank.org:act:
Type material. Goose Cove, Cedar Key, Florida, USA, 25.ii.2017 ( Holotype UF Mollusca 520997, Paratype LACM 3653, Paratype CASIZ 229210) collected by G. Paulay.
Type locality. Cedar Key , Florida, USA, 29.13326 N, 83.03748 W, inside cephalaspidean egg masses on low intertidal sand bar GoogleMaps .
Etymology. Named in honor of Jim Henson, creator of the muppets who educated and entertained generations of children while they ate their eggs for breakfast. As Kermit the Frog famously sang, “It’s Not Easy Bein’ Green,” a fitting allusion to the one lineage of sacoglossans that evolved away from herbivory among their many green relatives.
Additional material examined. United States, Florida, Cedar Key, Goose Cove : UF Mollusca 506367, intertidal sand spit, inside oval, cephalaspid egg masses; 12.ii.2017 [>10 spcs.] ; UF Mollusca 506376, 25.ii.2017 [~20 spcs.] ; UF Mollusca 506377, 25.ii.2017, egg masses laid in captivity .
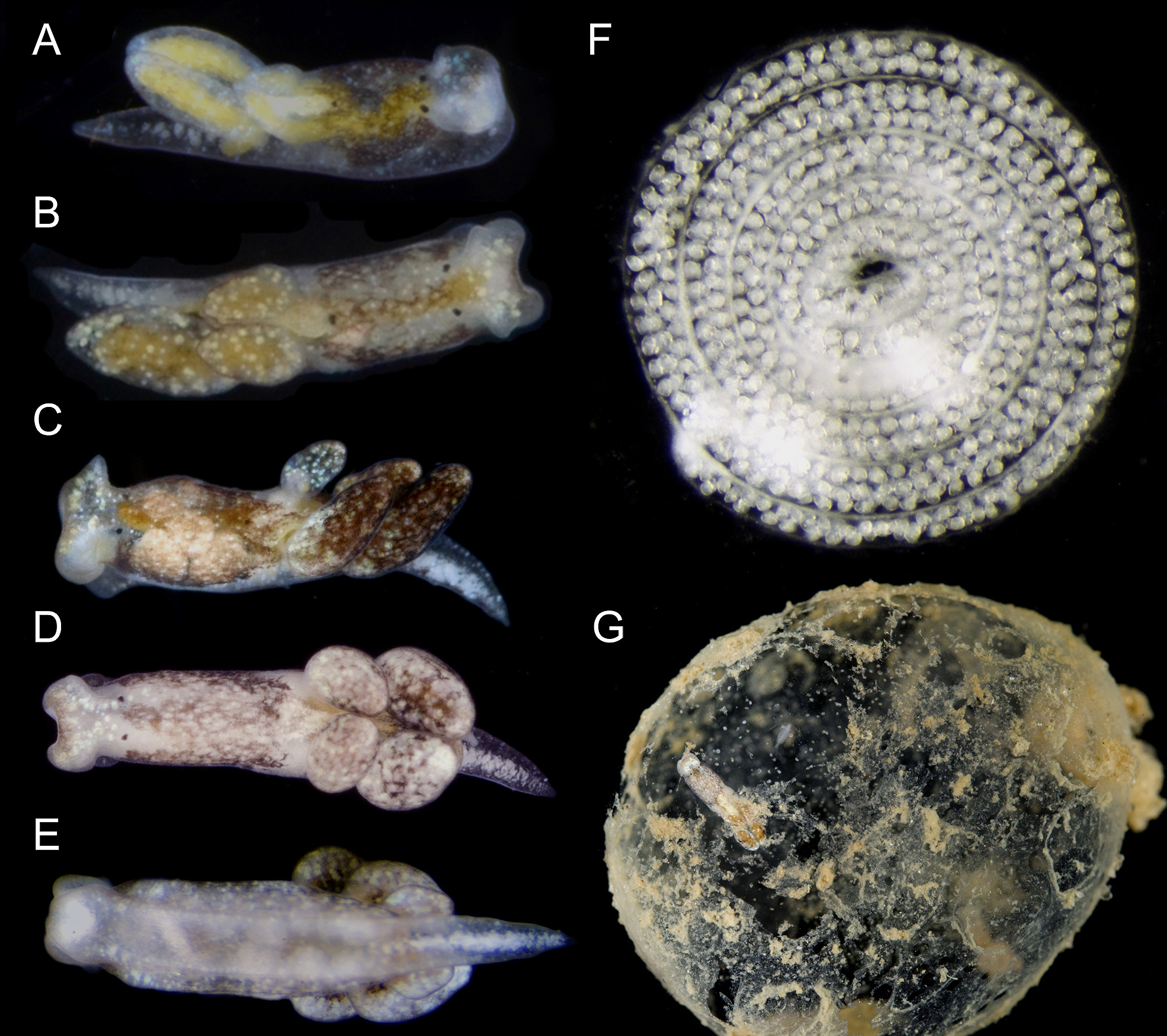
External morphology. Live animals up to 5 mm in length. Body smooth, elongated and tapering posteriorly to pointed tail (tl) ( Figs. 1 View FIGURE 1 , 4 View FIGURE 4 ). Translucent to creamy body with diffuse dark brown and yellowish blotches on dorsum ( Figs. 1 View FIGURE 1 A–D). White rounded glands distributed all over body, head and cerata (ct); larger bluish glands closer to posterior border of head ( Fig. 1A View FIGURE 1 ), and forming longitudinal, central row on tail ( Figs. 1C View FIGURE 1 , 4 View FIGURE 4 A–C). Dorsolateral brown patches and white glands still visible on preserved specimens, but digestive gland becomes whitish. Head square shaped, widest part of body, comprising ~¼ of body length ( Figs. 1A, B View FIGURE 1 , 4A View FIGURE 4 ). Rhinophores extremely re- duced to short expansions on posterior end of head. Eyes (ey) large, located dorsolaterally at posterior end of head ( Figs. 4 View FIGURE 4 A–B). Foot (ft) with longitudinal groove ( Figs. 1E View FIGURE 1 , 4C View FIGURE 4 ). Pericardial hump inconspicuous, positioned midcentrally on body just before anterior-most cerata. Dorsal vessels absent. Kidney opaque white, with renal aperture or nephrostome (np) marked by one black dot. Digestive gland yellowish. Cerata organized in two longitudinal rows, each row with three fusiform cerata ( Fig. 4E View FIGURE 4 ). Male aperture (mp) positioned on right side of body lateral to right eye ( Fig. 4E View FIGURE 4 ). Female aperture (fp) close and posterior to male aperture. Vaginal opening (vg) centrally positioned on right side of body, at same level as nephrostome, and far from female aperture ( Fig. 5A View FIGURE 5 ). Anus (an) positioned mid-dorsally on body, just posterior to eyes ( Fig. 5A View FIGURE 5 ).
Circulatory and excretory systems. Pericardium extremely small. Ventricle (vt) spherical and muscular, size ~5× bigger than auricle (au) ( Fig. 5B View FIGURE 5 ). Auricle inconspicuous and thin-walled with smooth surface. Kidney (kd) as thin, elongated, flat gland; unbranched; positioned posteriorly to pericardium, near base of auricle. Nephrostome readily visible and marked as a dark spot near pericardium.
Reproductive system. Gonad comprising multiple hermaphrodite follicles (hf) with irregular shape, slightly variable in size ( Fig. 5C View FIGURE 5 ). Multiple small ducts connect all follicles to one main hermaphrodite duct (hd) that expands forming one tubular ampulla (am). Ampulla occupies more than half of hermaphrodite duct. Hermaphrodite duct connects to male duct (md) proximally to ampulla expansion, and runs to oviduct on fertilization chamber (fc). Bursa copulatrix (bc) with its own aperture and one main duct connected to fertilization chamber; this organ was considered the vagina by Jensen (1996) but we use bursa copulatrix following Gascoigne (1975). Albumen gland (ag) highly branched over hermaphrodite follicles and with one connection to fertilization chamber at the same point as hermaphrodite ducts and one distal connection on bursa copulatrix duct. Genital receptacle (gr) as large as bursa copulatrix, also connected to fertilization chamber, closer to glandular oviduct (ov). Glandular oviduct with two distinct glands. Capsule gland (cg) innermost, larger, thicker and flimsy. Mucus gland (mg) next to female opening on right side of body, less translucent and firmer than capsule gland. Prostate gland (pr) formed by bilobed short gland, positioned anteriorly in animal body under intestine and over esophagus. Vas deferens (vd) long, ~3× longer than penis. Penis (pe) short and conical, with a terminal slightly curved stylet approximately 100 µm in length, about half as long as overall length of penis ( Figs. 6 View FIGURE 6 A–B).
Nervous system. Central nervous system (ns) composed of six ganglia, positioned posterior to buccal mass around anteriormost part of esophagus (es) ( Figs. 5 View FIGURE 5 D–E). Cerebro-pleural ganglia (cp) as large as pedal ganglia (pg), each ganglion with five main innervations ( Fig. 5D View FIGURE 5 ). One innervation highly ramified close to ganglion, running towards posterior end of head to reduced rhinophoral region. Optical nerve twice as long as cerebro-pleural ganglion. Cerebral commissure external, short and thin. Buccal ganglia (bu) half as large as supraintestinal ganglion (sp), with one main innervation running from each ganglion to anterior part of buccal mass. Buccal commissure internal. Pedal ganglia with four main innervations running towards posterior end of body attached to foot under all organs. Pedal commissure internal or reduced. Visceral ganglia composed of one large abdominal ganglion (ab), half the size of cerebral ganglion and with two innervations, and one supraintestinal ganglion slightly smaller than abdominal ganglia and with one innervation ( Fig. 5E View FIGURE 5 ). Short connection joins abdominal ganglion to supraintestinal ganglion; longer innervated connection joins abdominal ganglion to cerebro-pleural ganglion, same length as cerebral commissure ( Fig. 5E View FIGURE 5 ).
Digestive system. Buccal mass (bm) barrel-shaped, short, elongated, 3× longer than width ( Figs. 7 View FIGURE 7 A–B). Dorsal septate muscle (ds) with tiny muscular transversal bundles close to each other and hardly distinguishable. Oral sphincter reduced. Ascus musculature (ma) running ventrally from oral sphincter to middle part of buccal mass, composed of tiny longitudinal muscles holding the descending limb of buccal mass. Ascus either absent or extremely reduced. Radula with ascending limb composed of 11 barely recognizable teeth in formation ( Figs. 6 View FIGURE 6 C–D, 7C). Descending limb half the length of ascending limb, with well-formed leading tooth plus three rod-like teeth ( Fig. 7C View FIGURE 7 , tooth numbers 1–3). Leading tooth spur-shaped, ~ 15 µm long on two separately prepared radulae; similar in length to base of tooth ( Figs. 6 View FIGURE 6 C–D, 7C: tooth 4). Edge smooth and lateral flanges absent. Leading tooth most closely resembles a highly reduced blade-like shape among major categories of tooth shape (blade, sabot, triangular; Jensen 1997a,b).
Esophagus thin and elongated, length ~ 3x longer than buccal mass length and bit longer than intestine (in) length. Esophageal inner surface with one pair of tiny folds ( Fig. 7D View FIGURE 7 ). Salivary glands (sg) paired, attached on most anterior part of esophagus, cover first 1/5 of esophagus length. Esophageal pouch absent. Stomach (st) flat, wide, positioned on middle of body under albumen gland and some dorsal follicles; thin-walled with inner surface with no folds. Two elongated digestive gland (dg) ducts run towards posterior end of body ( Figs. 5A View FIGURE 5 , 7B View FIGURE 7 ). Each duct branches in three straight non-ramified ducts inside all cerata. Intestine elongated, thick, with inner surface covered with irregular ridges ( Fig. 7D View FIGURE 7 ).
Reproduction. Olea hensoni n. sp. was maintained in dishes in the laboratory for several days. After their host egg mass disintegrated, animals crawled around in the dish and on the surface tension, a habit also common in O. hansineensis . They laid whitish, spiral egg masses ranging from loosely to tightly coiled ( Fig. 1F View FIGURE 1 ). The egg ribbon was two embryos wide for the first (innermost) three whorls of one egg spiral, then was three embryos wide for the outermost two whorls.
Host ecology. Olea hensoni n. sp. was found on a large intertidal sand bar fronting a shallow bay on the Gulf coast of Florida, feeding in the gelatinous egg masses of an unidentified cephalaspidean ( Fig. 1G View FIGURE 1 ). Egg masses had developing veligers that hatched in the laboratory. No O. hensoni n. sp. were observed in any of the co-occurring, and similarly gelatinous, maldanid polychaete egg masses. Olea were found within the egg masses in manner similar to how O. hansineensis is found within the large, gelatinous egg masses of Melanochlamys diomedea ( Fig. 2C View FIGURE 2 ).
Distribution. Known only from the type locality in Cedar Key, Florida, U.S.A.
Remarks. In the original description, O. hansineensis was proposed to have affinity to the cladohepatic nudibranchs ( Agersborg 1923). The description noted that O’Donoghue hypothesized a separate family could be warranted to accommodate the morphological distinctiveness of O. hansineensis , for which the family Oleidae O’Donoghue, 1926 was subsequently erected. In O’Donoghue’s classification, some sacoglossan families were grouped with the cladohepatic nudibranchs, including Oleidae and ‘ Stiligeridae ,’ while the family ‘Hermaedidae’ was placed in a separate ‘Section Ascoglossa’. This system was rife with problems, including a non-monophyletic Sacoglossa , and the erroneous placement of numerous sacoglossan taxa; for instance, Aplysiopsis enteromorphae (Cockerell & Eliot, 1905) was classified as Phyllobranchopsis enteromorphae within the Stiligeridae , but actually belongs in Hermaeidae . Family Oleidae was later transferred to order Sacoglossa by Thiele (1931), whom Jensen (1996) gave as the authority for family Oleidae . Gascoigne (1975) synonymized Oleidae with Stiligeridae Iredale & O’Donoghue, 1923 . Jensen (1996) recognized that Limapontiidae Gray, 1847 had precedence over both Oleidae and Stiligeridae as the family name for ceratiform slugs including Olea . Our phylogenetic results support Jensen (1996) in keeping Olea within Limapontiidae , which would be rendered paraphyletic if the oophagous taxa were placed in a separate family.
Both molecular and morphological evidence confirm the new oophagous sacoglossan belongs in Olea . We recovered O. hensoni n. sp. as sister to O. hansineensis with significant support in BI analyses [PS = 0.95], although ML support was equivocal [BS = 53]. Externally O. hensoni n. sp. resembles O. hansineensis more closely than Calliopaea spp. Both O. hansineensis and O. hensoni n. sp. share a smaller number of cerata and extremely reduced rhinophores, whereas C. bellula has more cerata and much more pronounced rhinophores. External features similar to Olea were reported for smaller C. oophaga specimens ( Gascoigne & Sigurdsson 1977, fig. 1c) but the status of this species is uncertain, suggesting this could reflect the juvenile morphology of C. bellula .
The highly reduced radula of O. hensoni n. sp. was also comparable to that of O. hansineensis , comprising one spur-shaped active tooth of reduced size; an ascending limb of connected, cylindrical teeth with no obvious tip or cutting edge; and a descending limb with the same number of rod-shaped pre-radular teeth as reported for O. hansineensis ( Gascoigne 1975) . Only one fully formed tooth was present in all specimens of O. hensoni n. sp. analyzed, whereas Gascoigne (1975) reported intraspecific variation in the number of fully formed teeth in O. hansineensis . Also, the ascus in both Olea spp. is extremely reduced. In contrast, the radula of Calliopaea is similar to that of herbivorous sacoglossans, comprising ascending and descending limbs each with a row of fully formed, chisel-shaped teeth ( Gascoigne & Sigurdsson 1977; Gascoigne & Todd 1977). The radular morphology as well as genetic affinity for O. hansineensis both support the generic placement of O. hensoni n. sp. The absence of an esophageal pouch, described here for O. hensoni n. sp., was also previously reported for C. bellulla ( Gascoigne & Todd 1977) , but was not analyzed for O. hansinensis . This structure is observed in the great majority of Sacoglossa ( Jensen 1996) and is probably an adaptation for suctorial feeding on algae.
The reproductive system of O. hensoni n. sp. was similar in nearly all respects to that of O. hansineensis except that in O. hansineensis , no vaginal opening was detected; instead an internal bursa copulatrix was reportedly anchored to the right body wall, suggesting hypodermic insemination was necessary to effect transfer of allosperm ( Gascoigne 1975). In contrast, a vaginal pore was present on O. hensoni n. sp., and also on C. bellula ( Gascoigne & Todd 1977) . The penial stylet of O. hensoni n. sp. (~ 100 µm on a 2.5 mm long animal) was much more similar in size and shape to that of O. hansineensis , 120 µm long on a 6 mm long animal ( Gascoigne 1975), than to the elongated stylet of C. bellula ( 460 µm long on a 3 mm long animal; Gascoigne & Todd 1977). The bursa copulatrix of both Olea spp. is comparatively short compared to the elongated bursa in Calliopaea , which Gascoigne & Todd (1977) proposed was necessary to accommodate the proportionally longer penial stylet of C. bellula .
Although we are not aware of any modern records, Stiliger pusillus was described as externally similar to both Calliopaea and Olea , with comparable body coloration, short and simple rhinophores, and just three fusiform cerata on the posterior half of the body ( Baba 1959, plXXVII, fig. 1). Data on the internal morphology of this species are limited to the radula, which was figured with three well-formed teeth on the ascending limb and six on the descending limb; teeth were drawn as angled and pointed, similar to those of Calliopaea ( Baba 1959, pl XXVIII, fig. 1). Baba & Hamatani (1970) suggested S. pusillus should be transferred to Calliopaea , which would extend the range of this genus to span both Atlantic and Pacific Oceans like Olea . We concur that Calliopaea is likely the correct generic assignment for S. pusillus but formal reassignment should await either molecular data or a comprehensive revision of Calliopaea , including a reassessment of C. bellula versus C. oophaga .
Egg masses of O. hensoni n. sp. ( Fig. 1F View FIGURE 1 ) resembled those of O. hansineensis ( Fig. 2D View FIGURE 2 , and Agersborg 1923: pl. VI, fig. 4). Both species produce a flat egg ribbon two to three embryos wide, wound into a ‘watchspiral’. Such spiral egg masses are typical of some sacoglossan genera (e.g. Costasiella , Elysia ), but tight spirals are uncommon in family Limapontiidae , in which most genera produce sac-like egg masses or much wider spirals with few turns. The production of thin, spiral egg masses is another trait uniting O. hensoni n. sp. and O. hansineensis .
Both O. hansineensis View in CoL and Calliopaea View in CoL feed on a diversity of heterobranch eggs ( Crane, 1971; Jensen 1986), making it likely that O. hensoni View in CoL has a similarly broad diet. Because of their large, bulky nature, cephalaspid egg masses may be more easily entered by oophagous slugs than the narrower egg strings of other heterobranchs, and thus constitute preferred prey; alternatively, oophagous slugs may be more apparent within transparent cephalaspid egg masses and thus simply be more frequently observed feeding on cephalaspidean embryos. The sand bar at Cedar Key has a rich invertebrate fauna that includes diverse heterobranchs that could produce egg masses serving as suitable prey: Cerberilla tanna Marcus & Marcus, 1960 View in CoL , Spurilla braziliana MacFarland, 1909 View in CoL , Okenia aff. aspersa (Alder & Hancock, 1845) View in CoL , Haminoea succinea (Conrad, 1846) View in CoL , and Elysia cf. velutinus Pruvot-Fol, 1947 View in CoL ; several of these species can occur in large numbers, but none produces the type of egg mass in which O. hensoni View in CoL was found. The species producing the egg masses in which O hensoni View in CoL n. sp. was found were not encountered and attempts at barcoding the egg mass were not successful. The discovery of this species in a well-studied intertidal area underscores how poorly we know the marine biosphere.
| UF |
Florida Museum of Natural History- Zoology, Paleontology and Paleobotany |
| LACM |
Natural History Museum of Los Angeles County |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
SubClass |
Heterobranchia |
|
SuperOrder |
Sacoglossa |
|
Order |
|
|
Family |
|
|
Genus |
Olea hensoni
| Filho, Hilton Galvão, Paulay, Gustav & Krug, Patrick J. 2019 |
O. hensoni
| Filho & Paulay & Krug 2019 |
O. hensoni
| Filho & Paulay & Krug 2019 |
O hensoni
| Filho & Paulay & Krug 2019 |
Cerberilla tanna
| Marcus & Marcus 1960 |
Elysia cf. velutinus
| Pruvot-Fol 1947 |
Spurilla braziliana
| MacFarland 1909 |
Haminoea succinea
| Conrad 1846 |
Okenia aff. aspersa
| Alder & Hancock 1845 |
Calliopaea
| d'Orbigny 1837 |