Halosydna parva Kinberg, 1856
|
publication ID |
https://doi.org/10.11646/zootaxa.5032.2.1 |
|
publication LSID |
lsid:zoobank.org:pub:7A904156-23F6-4D88-9F8D-8E3683881F56 |
|
persistent identifier |
https://treatment.plazi.org/id/9E1D87E2-FFED-E65C-9CF8-809553D5FD98 |
|
treatment provided by |
Plazi |
|
scientific name |
Halosydna parva Kinberg, 1856 |
| status |
|
Halosydna parva Kinberg, 1856 View in CoL
Figures 9–16 View FIGURE 9 View FIGURE 10 View FIGURE 11 View FIGURE 12 View FIGURE 13 View FIGURE 14 View FIGURE 15 View FIGURE 16
Halosydna parva Kinberg 1856: 385 View in CoL .
Halosydna parva Hartman 1939: 33–34 View in CoL . Pl. 21, Figs 265–267. 1949: 18–19. Figs Pl. 1, Figs. 5–9 View FIGURE 5 View FIGURE 6 View FIGURE 7 View FIGURE 8 View FIGURE 9 .
Halosydna parva Salazar-Silva 2013: 36–39 View in CoL View Cited Treatment . Figs 17–18 View FIGURE 17 View FIGURE 18 .
Material examined. MUSM Nº 4647, 1 specimen, complete, Punta Blanca , Arequipa, Peru, 15°27’39.36”S 75°2’2.98”W, Station 2A, coll. from rocky shore at low tide under rocks, 14 September 2019, by D. Valencia-Soto and D. Valencia-Valencia, pharynx dissected and dissolved for complete jaw examination GoogleMaps . MUSM Nº 4648, 2 specimens, same sampling data, pharynges dissected for jaw examination . MUSM Nº 4649 (a), 1 specimen, Punta Blanca , Arequipa, Peru, 15°27’39.36”S 75°2’2.98”W, Station 2C, same sampling data, pharynx dissected for jaw examination GoogleMaps . MUSM Nº 4650, 2 specimens, Punta Blanca , Arequipa, Peru, 15°27’38.70”S 75°2’0.60”W, Station 3A, coll. from rocky shore at low tide under rocks, 14 September 2019, by D. Valencia-Soto and D. Valencia-Valencia, pharynges dissected for jaw examination GoogleMaps . MUSM Nº 4665, 6 specimens, Punta Blanca , Arequipa, Peru, 15°27’38.70”S 75°2’0.60”W, Station 3C, same sampling data, pharynges dissected for jaw examination GoogleMaps .
Four specimens ( MUSM Nº 4649b–e) fixed using absolute ethanol, for molecular studies.
Description. Based on specimen MUSM N° 4647. Individual 22.3 mm long (from tip of median antennae to tip of anal cirri) and 4.4 mm wide with chaetae ( 3.4 mm without chaetae). Body with 36 segments, short, slender, flattened dorsoventrally and rectangular in cross-section ( Fig. 9A–B View FIGURE 9 ). Prostomium rounded, bilobed, broader than long, with three antennae antero-terminally ( Fig. 9C View FIGURE 9 ). Median ceratophore stout, with dark pigment, placed in anterior notch of prostomium; lateral ceratophores as anterior projections of prostomium, with dark pigment and thinner than median ceratophore ( Fig. 9C View FIGURE 9 ). Ceratostyles of all antennae with subdistal swellings and filiform tips; lateral ceratostyles shorter than median ceratostyle ( Fig. 9C View FIGURE 9 ). Two pairs of dark eyes placed dorsolaterally: anterior pair slightly anterior to widest part of prostomium and posterior pair on posterior part of prostomium ( Fig. 9C View FIGURE 9 ). One of two palps present, conical, smooth, with subdistal swelling and filiform tip ( Fig. 9C View FIGURE 9 ). Facial tubercle rounded, with dark pigmentation.
Tentaculophores lateral to prostomium, cylindrical with two chaetae each. Tentacular cirri resemble median ceratostyle ( Fig. 9C View FIGURE 9 ). Dorsal tentacular cirri longer than ventral tentacular cirri. Buccal segment with pair of inconspicuous rounded nuchal nodules; nuchal fold absent ( Fig. 9C View FIGURE 9 ). Buccal cirri with same appearance of lateral antennae. Dark-pigmented bands present at base and near subdistal swelling of ceratostyles, tentacular and buccal cirri. Dorsal cirri with similar appearance of tentacular cirri ( Fig. 9A–B View FIGURE 9 ). Dorsal tubercles nodular, inconspicuous ( Fig. 9A View FIGURE 9 ). Dorsum with dark-pigmented transverse bands on posterior region of each segment ( Fig. 9A View FIGURE 9 ).
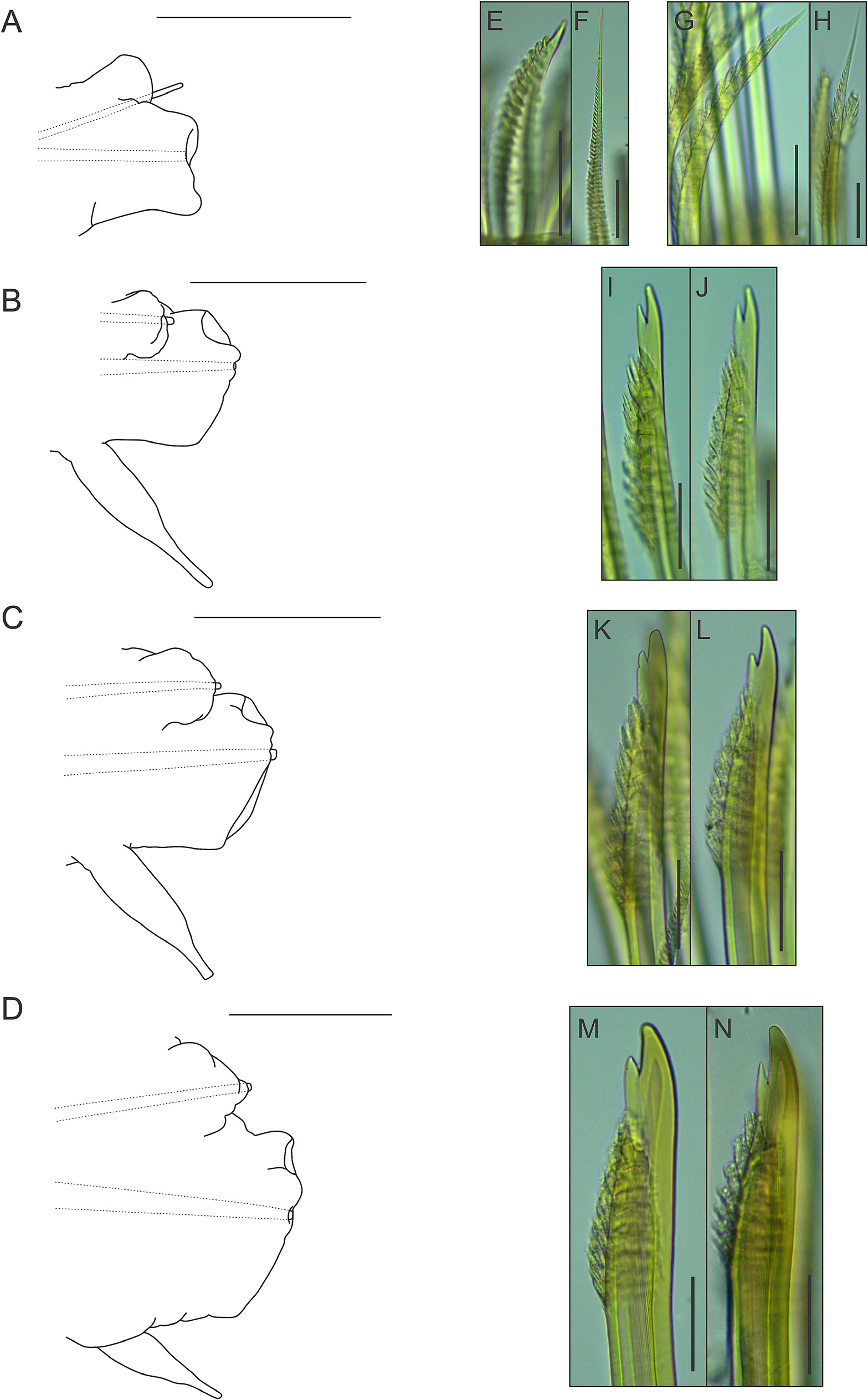
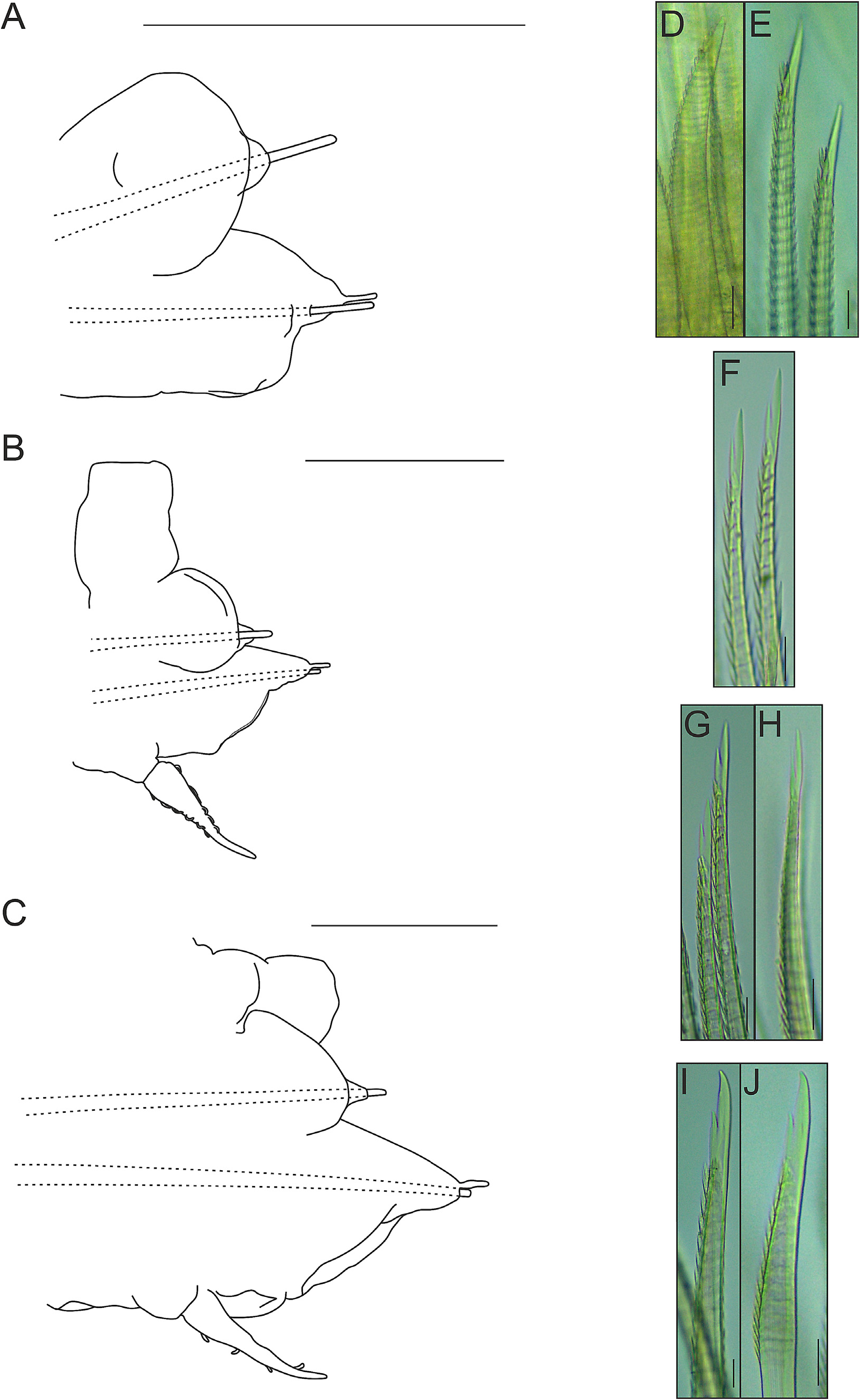
Parapodia with small, somewhat conical notopodia placed anteriorly (except for first parapodium, where notopodium is placed posterior to neuropodium) ( Fig. 10A–D View FIGURE 10 ), with indistinct chaetal lobes. Well-developed neuropodia not deeply incised dorsally and ventrally, with indistinct chaetal lobes; prechaetal lobe distally truncate, with small rounded lobe over tip of acicula; postchaetal lobe distally truncate, with similar length (except for first parapodium, where both chaetal lobes are somewhat concave distally) ( Fig. 10A–D View FIGURE 10 ). Neuropodial supra-acicular process and terminal papilla absent. Both rami with aciculae penetrating epidermis ( Fig. 10A–D View FIGURE 10 ). Ventral cirri of anterior parapodia slightly longer than those present in parapodia of median region of the body, with bulbous bases, filiform tips and smooth surfaces ( Fig. 10A–D View FIGURE 10 ). Nephridial papillae present from 8 th segment, with blunt tips.
Notochaetae arranged in short bundles, with numerous transverse rows of spines: blunt-tipped notochaetae ( Fig. 10E View FIGURE 10 ), in superior position and capillary notochaetae ( Fig. 10F View FIGURE 10 ), in inferior position. Notochaetae become longer from superior to inferior positions. Neurochaetae stouter than notochaetae, falcate, subdistally thickened, with subdistal rows of spines; rows of spines larger distally than basally. First parapodium with entire-tipped neurochaetae ( Fig. 10G–H View FIGURE 10 ); remaining parapodia with bidentate neurochaetae ( Fig. 10I–N View FIGURE 10 ). Pygidium with two anal cirri ( Fig. 9B View FIGURE 9 ).
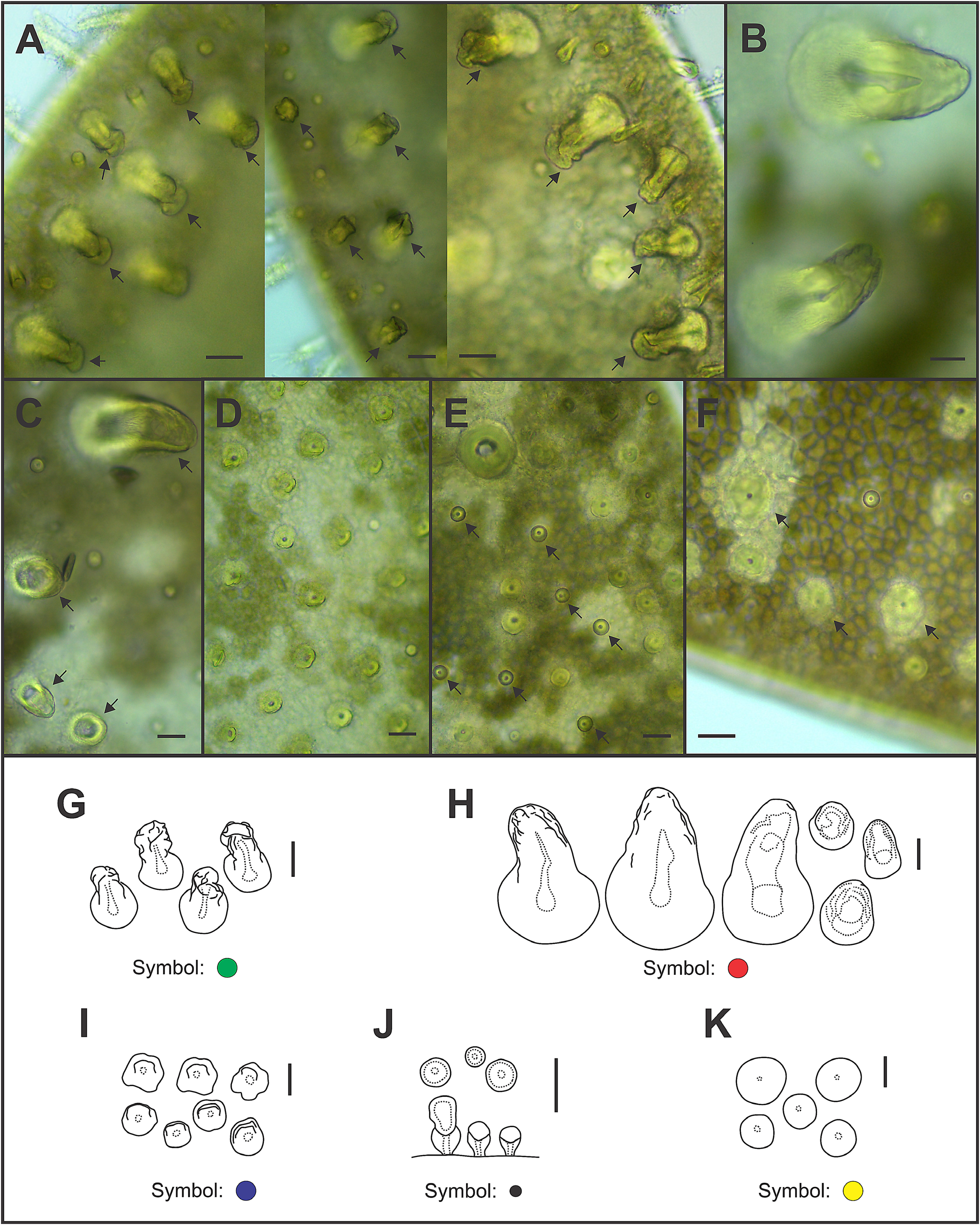
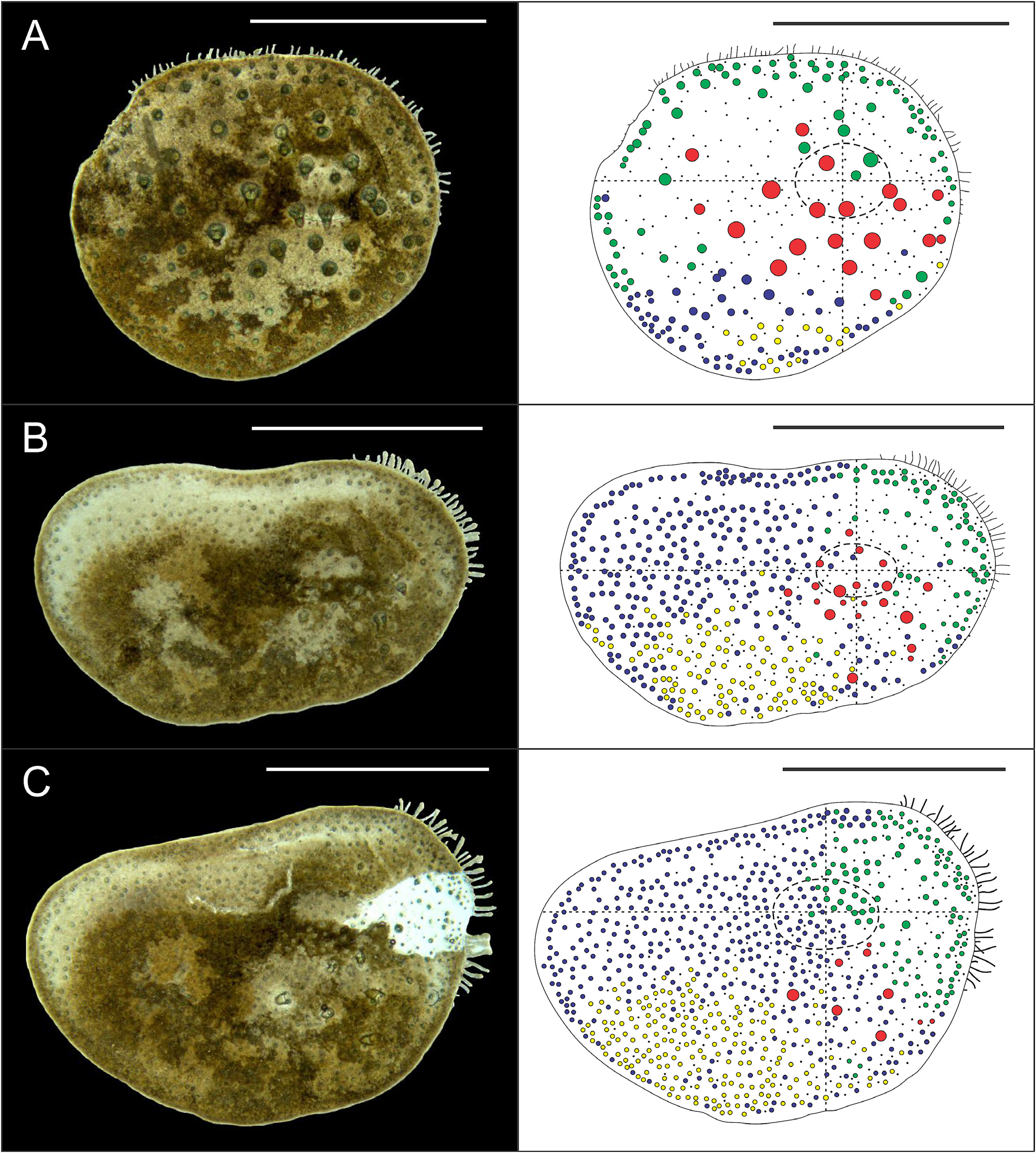
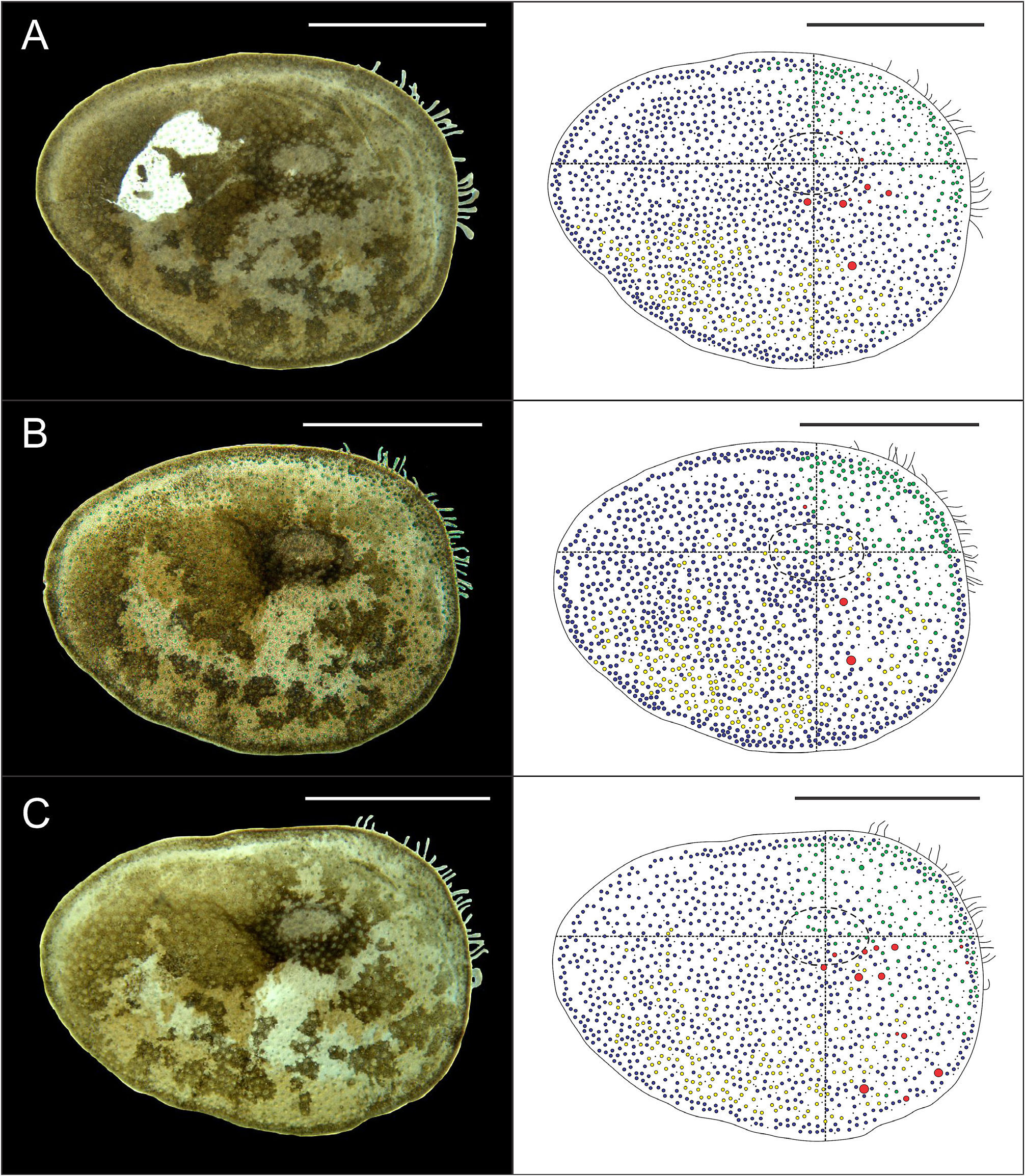
Eighteen pairs of elytra on segments 2, 4, 5, 7, 9, 11, 13, 15, 17, 19, 21, 23, 25, 27, 28, 30, 31, 33; covering all the body, leaving neurochaetae and dorsal cirri exposed. Last three segments non-elytrigerous, covered by last pair of elytra. First pair of elytra slightly rounded ( Fig. 12A View FIGURE 12 ), second pair reniform ( Fig. 12B View FIGURE 12 ), median and posterior slightly oval ( Figs 13 View FIGURE 13 , 14A–B View FIGURE 14 ) and last pair triangular ( Fig. 14C View FIGURE 14 ). All elytra provided with marginal fringe of papillae commonly arranged on AI and AIII, white margins mainly present on AI and AII and a white spot over the elytrophore scar ( Figs 12–14 View FIGURE 12 View FIGURE 13 View FIGURE 14 ). Remaining surfaces randomly mottled with white and dark spots ( Figs 12–14 View FIGURE 12 View FIGURE 13 View FIGURE 14 ). Elytrophore scars oval.
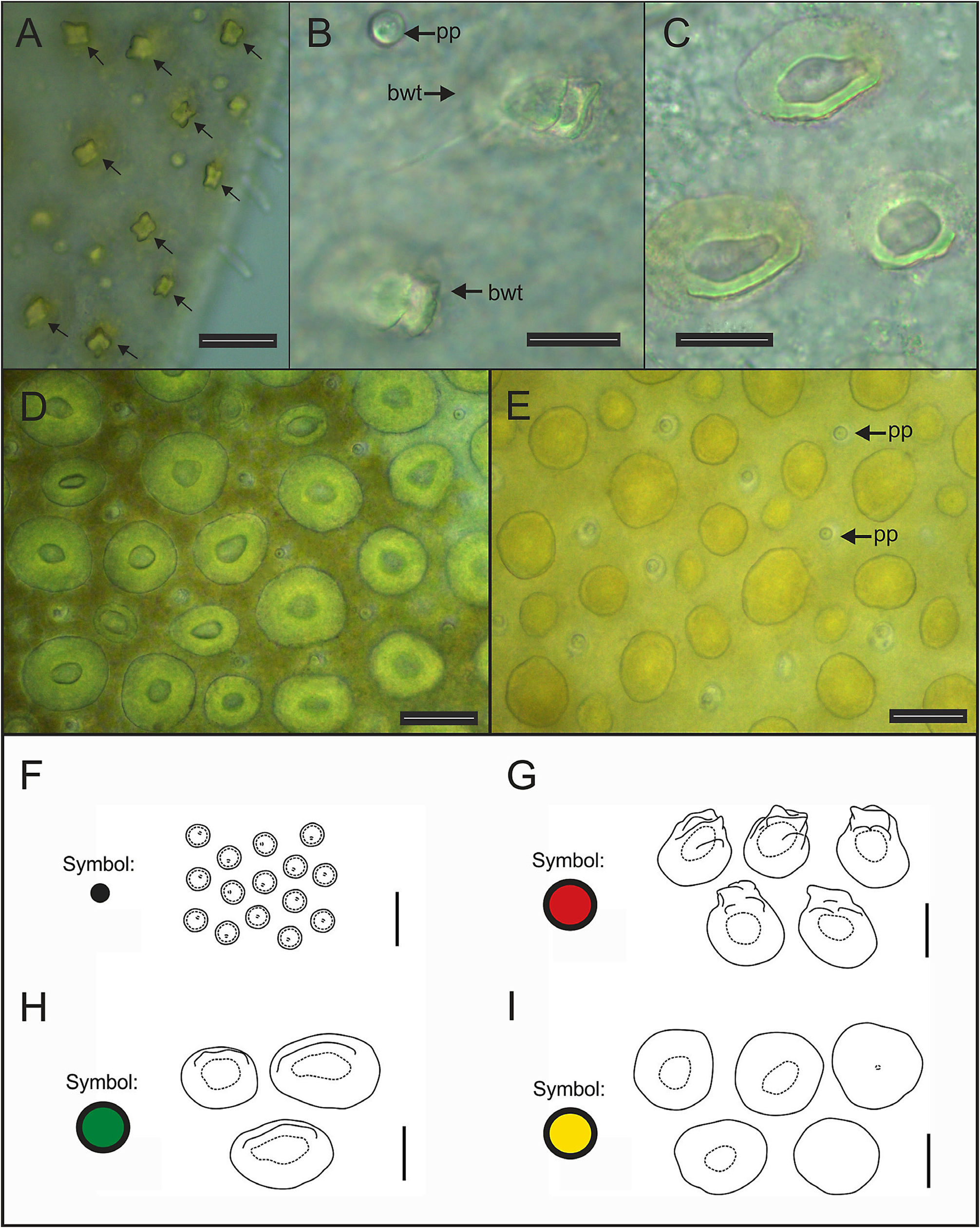
All elytral surfaces are covered by rounded translucent papillae (seen from above) with narrow bases, sometimes provided with a short filiform tip ( Fig. 11E, J View FIGURE 11 ), mainly on AI and AIII ( Fig. 12–14 View FIGURE 12 View FIGURE 13 View FIGURE 14 ) and four kinds of hardwalled tubercles: (a) conical microtubercles with swollen, wrinkled tips, sometimes bifid (slightly quadrangular when seen from above) ( Fig. 11A, G View FIGURE 11 ) mainly on AI, some on AII and AIII ( Figs 12–14 View FIGURE 12 View FIGURE 13 View FIGURE 14 ); (b) conical macrotubercles with smooth and rugose tips, some with wide internal concavities ( Fig. 11B, C, H View FIGURE 11 ), mainly on AI, AIII and AIV ( Figs 12–14 View FIGURE 12 View FIGURE 13 View FIGURE 14 ); (c) truncate microtubercles ( Fig. 11D, I View FIGURE 11 ) on AII, AIII and AIV ( Figs 12–14 View FIGURE 12 View FIGURE 13 View FIGURE 14 ) and (d) flattened microtubercles ( Fig. 11F, K View FIGURE 11 ) on AIII and AIV ( Figs 12–14 View FIGURE 12 View FIGURE 13 View FIGURE 14 ). Tubercle morphology (both macro- and micro-) changes gradually depending on location, giving rise to transitional forms between zones where each tubercle type predominates.
Like shape, dimensions of both macro- and microtubercles change gradually over the elytral surface depending on location. Except for first pair of elytra, macro- and microtubercles located on AI and AIII are taller than those present on AII and AIV while macro- and microtubercles bases are slightly wider when located on AII, AIV and part of AIII. Both height and width of all macro- and microtubercles decrease gradually from anterior to posterior pairs of elytra; however, dimensions of conical macrotubercles vary regardless of this pattern ( Fig. 15 View FIGURE 15 ). The translucent rounded papillae (the smallest elytral ornamentation) tend to vary in width; however, a pattern between their location over the elytral surface and their width was not observable.
Pharynx with nine pairs of anterior terminal papillae ( Fig. 16A View FIGURE 16 ). Two pairs of reddish, chitinous, hollow and asymmetric jaws, posterior to papillae ( Fig. 16B–C View FIGURE 16 ). Longer fangs present in dorsal right jaw and ventral left jaw ( Fig. 16B–C View FIGURE 16 ). Denticles absent from jaws ( Fig. 16 View FIGURE 16 ).
Variation. Collected individuals have 36 body segments and range between 13.6–22.3 mm long and 3.1–5 mm wide (including chaetae). Of all 12 examined specimens, only 3 were ovigerous. Minimum size for ovigerous specimens was 13.6 mm long; however, longer non-ovigerous individuals were observed. Significant morphological differences between non-ovigerous and ovigerous individuals were not observed.
Individuals exhibit some intraspecific variability such as: (1) a short, triangular acicular lobe in neuropodia; (2) distinct degrees of development of both neuropodial lobes and nuchal nodules and (3) presence of an inconspicuous nuchal fold. These variations could not be correlated to size since these were present regardless of this parameter.
Elytral asymmetry, which could be caused by damage/loss and replacement of elytra (A. Murray, pers. comm.), was observed in all examined individuals as well. It was restricted to the conical macrotubercles, especially those with wide internal concavities. Elytral asymmetry is here classified in three categories: (a) according to their presence (i.e., right elytron may have them while the left elytron not and vice versa); (b) according to their dimensions (i.e., height and width of conical macrotubercles can vary between left and right elytra of a single pair) and (c) according to their number (i.e., right elytron may have several conical macrotubercles while the left elytron only one, two or a few and vice versa).All elytral asymmetry categories can be present in any elytral pair, even simultaneously (except for the first kind).
Complete absence of conical macrotubercles with wide internal concavities in a whole, single pair of elytra (i.e., both left and right elytra) was observed as well, in the first pair of elytra of specimen MUSM N° 4648b. Furthermore, some individuals exhibited corrugated elytral surfaces with conspicuous elytral ornamentations (i.e. taller and wider than other specimens) but their morphology and distribution over the elytral surface remained the same.
Remarks. The specimens agree with descriptions by Hartman (1949) and Salazar-Silva (2013) based on type material of Halosydna parva Kinberg, 1856 . However, some details should be highlighted. Hartman (1949) reexamined the type specimens of Halosydna parva Kinberg, 1856 and stated this species had elytra with marginal papillae (absent in larger individuals), “large, low, chitinous cones on the posterior half of the surface” in the first twelve pairs of elytra, “minute pickles” in the remaining elytra (or smooth) and bidentate neurochaetae. Later, Salazar-Silva (2013) designated a lectotype and pointed out this species had elytra with marginal papillae (but absent in posterior ones), conical microtubercles, distally rounded macrotubercles (but absent in the first pair of elytra) and bidentate neurochaetae.
Both descriptions have some differences regarding elytral characters but these may be explained. According to Salazar-Silva (2013), the lectotype is in poor condition so absence of marginal papillae in posterior pairs of elytra may be an artifact due to poor procedures of preservation and age of the individual (A. Murray, pers. comm.). This could also be the case for larger individuals without marginal papillae referred by Hartman (1949). On the other hand, absence of conical macrotubercles in the first pair of elytra described by Salazar-Silva (2013) agrees with the elytral asymmetry recorded for the individuals herein examined. Comparison between Halosydna parva Kinberg, 1856 and other Halosydna species recorded for the Pacific Coast of South America can be found in Table 4.
Distribution. The type locality is Chincha Islands, Peru ( Kinberg 1856). Also recorded for Cañete ( Tasso et al. 2018), Lima ( Paredes et al. 1999) and Independencia Bay ( Hartman 1939), Peru; Ecuador (La Plata Island; Albemarle Island, Galapagos) ( Hartman 1939). Herein, Halosydna parva is newly recorded for Punta Blanca, Arequipa, Peru.
Ecology. Halosydna parva Kinberg, 1856 has been recorded inhabiting a range of hard bottom environments at depths between 0–22 m, in intertidal environments and coral ( Hartman 1939, Tasso et al. 2018; Paredes et al. 1999). Herein, individuals of Halosydna parva Kinberg, 1856 were found alone or accompanied by specimens of the same species. As far as was observed, neither Lepidonotus aff. crosslandi peruana Hartmann-Schröder, 1962 and Harmothoe aff. hirsuta Johnson, 1897 shared the same habitat (i.e. inferior surfaces of rocks) with Halosydna parva Kinberg, 1856 .
tized” is the description attributed to that author and means “hard-walled”.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Halosydna parva Kinberg, 1856
| Valencia-Soto, David 2021 |
Halosydna parva
| Hartman, O. 1939: 34 |
Halosydna parva
| Kinberg, J. G. H. 1856: 385 |