Asaccotrema vietnamiense, Sokolov & Gordeev, 2019
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4674.4.4 |
|
publication LSID |
lsid:zoobank.org:pub:BD3A0EE3-1F60-468B-9793-5E5EE55C6EBE |
|
DOI |
https://doi.org/10.5281/zenodo.5619668 |
|
persistent identifier |
https://treatment.plazi.org/id/23247B89-2433-4CAD-9247-7648C9AFF36D |
|
taxon LSID |
lsid:zoobank.org:act:23247B89-2433-4CAD-9247-7648C9AFF36D |
|
treatment provided by |
Plazi |
|
scientific name |
Asaccotrema vietnamiense |
| status |
sp. nov. |
Asaccotrema vietnamiense n. sp.
urn:lsid:zoobank.org:act:23247B89-2433-4CAD-9247-7648C9AFF36D
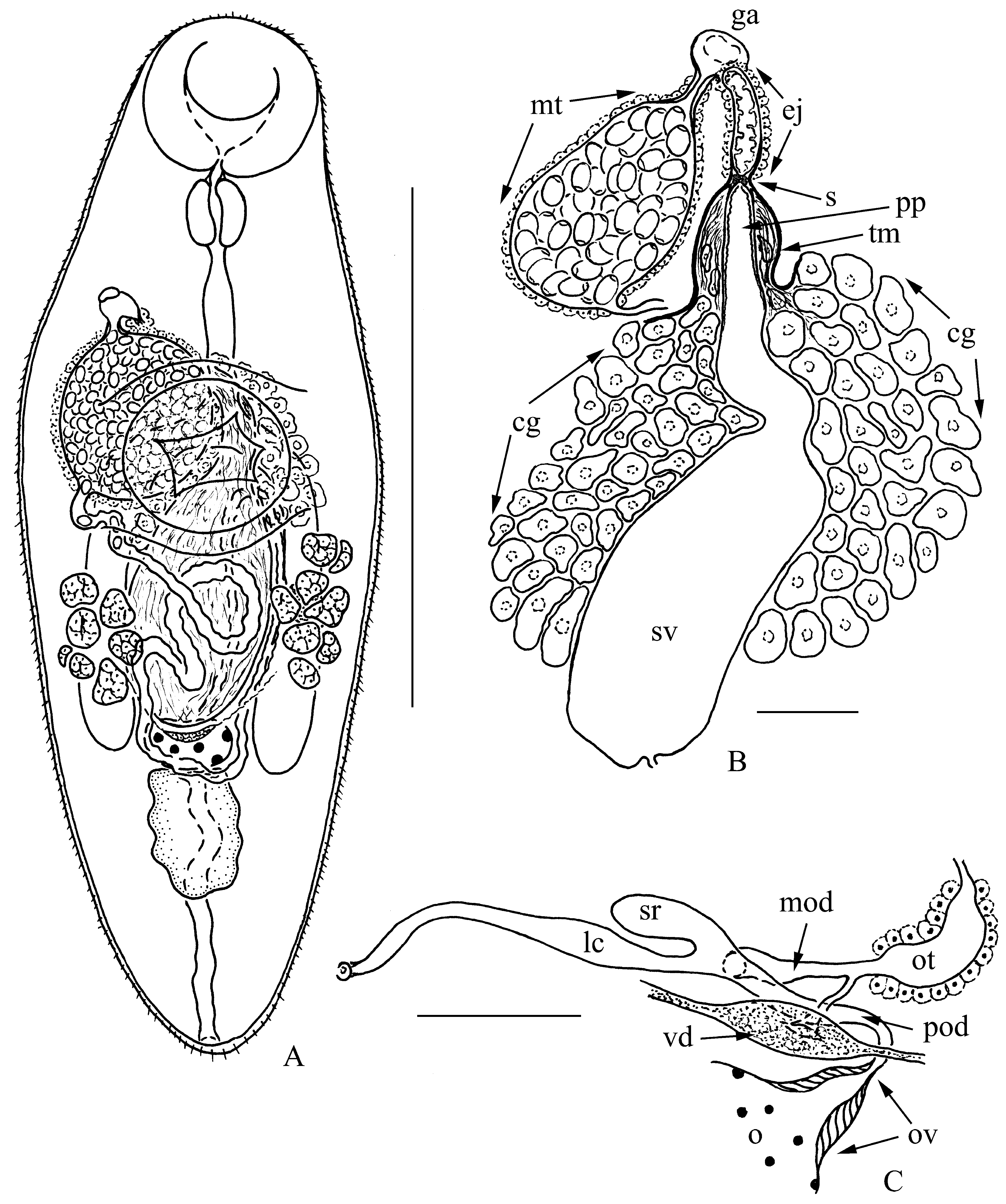
( Fig. 1 View FIGURE 1 )
Type host: Rasbora paviana Tirant (Actinopterygii: Cyprinidae ).
Type locality: The brook Da Brout in the Cat Tien National Park , Vietnam (11°26′34″N; 107°25′45″E) GoogleMaps .
Site of infection: Liver.
Deposited material: Holotype ( IPEE RAS #1314 View Materials ) and paratypes (3) IPEE RAS # 1314–1316 View Materials (whole-mounted excysted progenetic metacercariae), v oucher specimens (3) with isolated terminal genitalia.
Zoobank Life Science Identifier: ( LSID) for Asaccotrema vietnamiense n. sp. is urn:lsid:zoobank.org:act:23247B89-2433-4CAD-9247-7648C9AFF36D .
Prevalence: 1 of 1 host.
Intensity: 8 worms/host specimen.
Description: (Based on 7 specimens). Progenetic metacercariae. Body elongate-oval, length 775–875 [800], maximum width 275–350 [288] in middle third of body. Forebody 33.3–38.7 [34.4]% of body length, occasionally (in one of four specimens) 45.7 %. Tegument spinous. Oral sucker rounded, 125–138 × 138–150 [125 × 138]. Ventral sucker rounded, 125–150 × 138–163 [125 × 138]. Oral sucker to ventral sucker width ratio 1: 0.92–1.08 [1: 1]. Mouth opening subterminal. Prepharynx very short. Pharynx muscular, 55–61 × 49–61 [55 × 49], esophagus 122–159 [128]; intestinal bifurcation at level of anterior part of ventral sucker or just anterior to anterior edge of ventral sucker, occasionally (in one of four specimens) at some distance anterior to ventral sucker. Ceca extending to anterior end of testis. Testis single, slightly irregular, median, 73–153 × 55–67 [92 × 67], postovarian, in middle part of hindbody. Cirrus-sac absent. Seminal vesicle saccular proximally and tubular distally, surrounded by numerous prostate gland-cells; extending to ovary, occasionally (in one of four specimens) to midlevel of ventral sucker. Pars prostatica tubular, not clearly separated from seminal vesicle, penetrated by ducts of prostate gland-cells, and terminating with sphincter. Main mass of prostate gland-cells lies free in parenchyma. Terminal portion of prostate gland-cells cluster, which includes ducts of prostate gland-cells and distal gland-cells, covered with thin-walled open-ended membrane. Distal end of membrane connected to proximal edge of wall of ejaculatory duct. Ejaculatory duct long, covered with gland-cells; communicating with common genital atrium. Inner cytoplasmic layer of wall of ejaculatory duct forms numerous finger-like protrusions. Common genital atrium distinct. Genital pore dextrosublateral, at about of midlevel of esophagus. Ovary entire, transverse-oval, median, 31–49 × 61–73 [43 × 61], in middle part of hindbody, contiguous with testis, exceptionally (in one of four specimens) at level of posterior edge of ventral sucker and distinctly separated from testis. Muscular ovicapt distinct. Laurer’s canal opens in dorsal pore at level of ovary. Canalicular seminal receptacle conspicuous, sacсular. Oviduct connects to common vitelline duct before becoming oötype. Common vitelline reservoir ventral to ovary. Oötype enclosed in Mehlis’ gland opens into uterus. Uterine seminal receptacle not detected. Vitellarium follicular; subglobular follicles in two short lateral preovarian fields of 7–9 each, lying in anterior half of hindbody, exceptionally (in one of four specimens) overlapping ovary and ventral sucker to some extent. Uterine coils between ventral sucker and posterior end of testis. Metraterm well-developed, ventral to ejaculatory duct, rather dilated and filled with numerous eggs. Eggs numerous, ovate, operculate, 15 × 10–12. Excretory vesicle tubular, extending to anterior edge of testis; excretory pore subterminal at posterior extremity.
Etymology: The specific epithet ‘ vietnamiense ’ is intended to mark the country ( Vietnam) where this species was found for the first time.
Remarks: Many features, like living in a freshwater environment, the spinous tegument, the sublateral genital pore, the follicular vitellarium organized in two short symmetrical fields, rather short intestinal branches, the presence of one testis and its position allow us to place A. vietnamiense n. sp. in the subfamily Asymphylodorinae , Lissorchiidae ( Bray 2008) .
This subfamily is currently composed of four genera: Asymphylodora Looss, 1899 s. lato, Brahamputrotrema Gupta, 1955 , Prosovitellina Wang, 1985 and Wangxiyunia Bray, 2008 ( Bray 2008) . Representatives of all these genera, like all the other lissorchiids (members of the subfamily Lissorchiinae ), have a well-developed cirrus-sac and their genital pore is positioned approximately at the level of the ventral sucker. In contrast, Asaccotrema vietnamiense n. sp. does not have a cirrus-sac, and the genital pore is positioned near the midlevel of the esophagus.
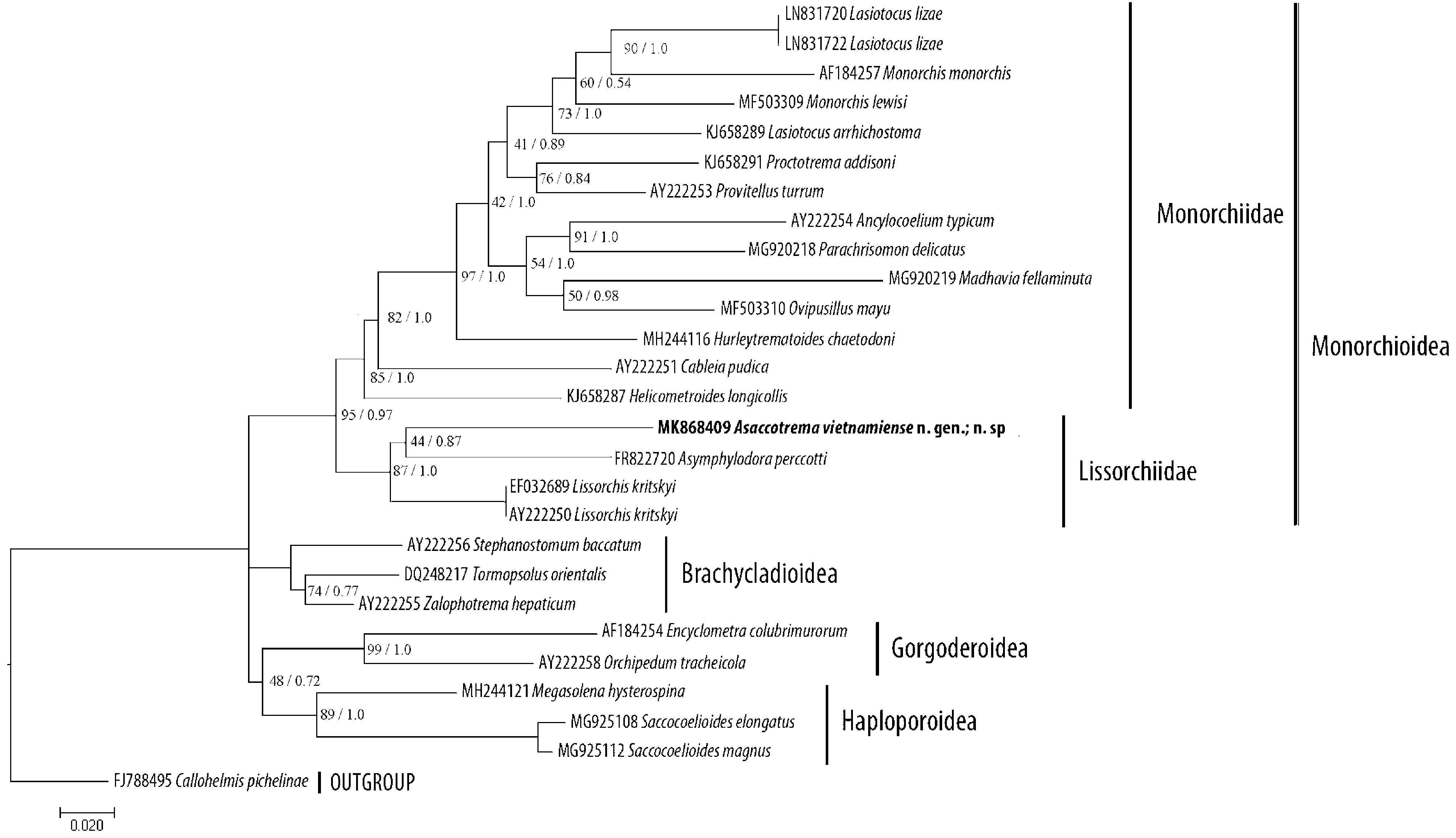
Phylogenetic position, PCR amplification of the 28S rRNA gene produced a 1300-bp fragment for the A. vietnamiense n. sp. specimen. After assembly and alignment procedures were carried out, the resulting 28S rRNA gene sequence from these species was 1135 bp long.
In both the ML and BI analyses, A. vietnamiense n. sp. appears as a member of the well-supported Lissorchiidae clade, which also includes Lissorchis kritskyi Barnhart & Powell, 1979 and Asymphylodora perccotti Besprozvanykh, Ermolenko & Atopkin, 2012 ( Fig. 2 View FIGURE 2 ). However, the phylogenetic relationships between representatives within the Lissorchiidae clade are poorly resolved.
Discussion. Among the lissorchiids progenesis at the metacercarial stage is not a unique feature of the species of concern, since this phenomenon is a characteristic of a number of representatives of the genus Asymphylodora Looss, 1899 sensu lato. The terminal genitalia and other reproductive organs of progenetic metacercariae of Asymphylodora spp. are morphologically identical to those in the adult stage (Serkova & Bychowsky 1940; Biguet et al. 1956; Stunkard 1959; Goodman & Panesar 1976; Kulakova 1982; Kudlai 2010). The terminal genitalia in the nonprogenetic metacercariae of Asymphylodora spp. and other lissorchiids are non-functioning, but structurally comparable with the those of the adult worms ( Macy & English 1975; Filimonova & Shalyapina 1979; Besprozvannykh 2005; Shimazu 2016). Metacercariae of Neopaleorchis catostomi Schell, 1973 are an exception, but only because in this species the cirrus-sac and metraterm are present as poorly differentiated primordia ( Schell 1973). In view of the above-mentioned facts, we have no doubt that the progenetic metacercariae of A. vietnamiense n. sp. demonstrate a definitive morphology for the terminal genitalia, typical of the adult hermaphroditic generation of this species. Such a morphology with respect to the terminal genitalia, unique for lissorchiids, allows us to describe these specimens not only as a new species, but as a new genus. The description A. vietnamiense n. sp. means an amendment to the diagnosis of the Lissorchiidae is required.
| IPEE |
A.N. Severtzov Institute of Ecology and Evolution, Russian Academy of Sciences |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |