Phyllomedusa neildi, Barrio-Amorós, César L., 2006
|
publication ID |
https://doi.org/ 10.5281/zenodo.173822 |
|
DOI |
https://doi.org/10.5281/zenodo.5665358 |
|
persistent identifier |
https://treatment.plazi.org/id/9671FC29-FFC5-4A34-FEFF-FDCBB760E36B |
|
treatment provided by |
Plazi |
|
scientific name |
Phyllomedusa neildi |
| status |
sp. nov. |
Phyllomedusa neildi sp. nov.
( Fig. 1 View FIGURE 1 , 2 View FIGURE 2 )
Holotype: MBUCV 6684, adult male, from the vicinity of Murucusa, Municipio Petit (11º 02’ N, 69º 35’ W), 550 masl, spurs of Sierra de San Luís, Estado Falcón, Venezuela, collected by C. Morón in August 1994.
Paratypes: CVULA 6500, 6502, adult males; CVULA 6501, 6503, adult females; EBRG 4754–5, adult males; MBUCV 6685–6, adult males (6685 cleared and stained). All with the same data as the holotype.
Referred material: MHNLS 1503 Estado Falcón: Curimagua, distrito Petit.
Etymology: The specific name is a patronym for Andrew Neild, lepidopterologist associated with the Natural History Museum of London ( UK) in recognition of his productive work in Venezuela ( Neild 1996).
Diagnosis: A member of the genus Phyllomedusa sensu stricto (Gonçalves da Cruz 1990; Faivovich et al. 2005) and of the Phyllomedusa tarsius group (defined below), with the following combination of characters: (1) moderate size (= 59.8 in males; = 73.3 in females); (2): snout of males strongly sloping in lateral profile, rounded to truncate in females; (3) Finger I longer than, and opposable to FII; (4) parotoid glands not apparent; (5) dentigerous processes of vomers present; (6) calcars and dermal appendages absent; (7) palpebral membrane not reticulated; (8) iris golden with black reticulations in life; (9) dorsal coloration green; concealed surfaces of hind limbs pink with white transverse bars or spots.
Comparison with other species: Compared with other species in the Phyllomedusa tarsius group, P. neildi is similar to P. trinitatis , P. tarsius and P. venusta , but can be easily distinguished by its significantly smaller size; SVL in Phyllomedusa trinitatis ( Fig 3 View FIGURE 3 ) males is from 70 to 81 mm (= 76.3); in females is from 90 to 95.5 (= 92 mm) [own data]; SVL in Phyllomedusa tarsius ( Fig 4 View FIGURE 4 ) ranges in males from 81 to 90 mm (= 84.1 mm); in females from 99.1 to 111.8 mm (= 104.0 mm) ( Duellman 1974); SVL in Phyllomedusa venusta ranges in males from 69 to 86.3 mm (= 77.4 mm); the mean in females is 97.7 mm ( Duellman and Trueb 1967, and own data combined). On the other hand, P. neildi has the concealed surfaces of the hind limbs coloured with white transverse bars or spots on a pink background (absent or very ill defined in other species). Phyllomedusa venusta has long and prominent parotoid glands (absent or not well developed in P. neildi ). Males of P. coelestis , a rare species in the upper Amazon of Peru and Ecuador, are similar in size to those of P. neildi (53.3–64.8 mm; Duellman and Mendelson III 1995), but P. neildi also differs from P. coelestis in coloration of the flanks and concealed surfaces of hind limbs. Phyllomedusa boliviana and P. c a m b a from the south western Amazon Basin lack a golden iris with black reticulation and have conspicuous parotoid glands.
Description of the type series: Phyllomedusa of moderate size (males 55.2–63.8,
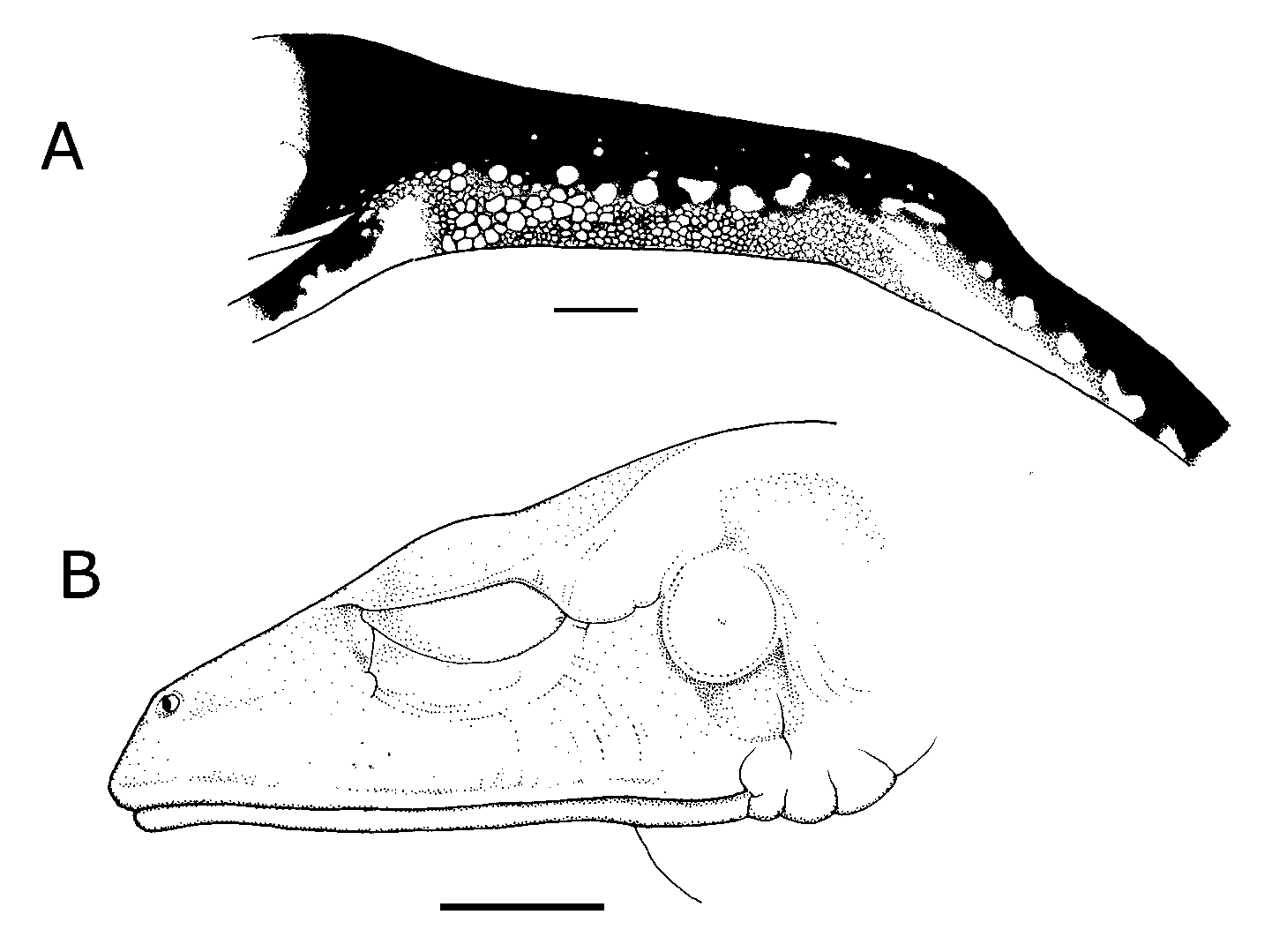
59.8; females 70.5–76, 73.2). Head ( Fig. 5 View FIGURE 5 B) longer than wide; top of head flat; snout short, oval in males, rounded in females in dorsal view; sloping in males, and rounded to truncate in females in lateral profile; canthus rostralis rounded; loreal region slightly concave; lips thin, not flared; nostrils not protuberant, directed laterally; internarial region flat; eyes protuberant; palpebral membrane transparent; parotoid glands indistinct; supratympanic fold apparent only in females, barely developed; tympanum vertically oval, distinct, except dorsally, hidden by supratympanic fold. Tongue enormous, round or cordiform, ½ to 2/3 free distally; maxillary teeth present; dentigerous processes of vomers small, transverse between choanae, separated by a distance equal to 2/3 of one process; each bearing 2–6 teeth (usually 4–6); vocal slits absent; vocal sac single, medial, not distinct.
Axillary membranes absent; arms slender, forearms moderately robust in males; ulnar fold indistinct; indistint row of protuberances, in some specimens; relative length of fingers I<II<IV<III; finger discs approximately 2/3 of TD; palmar tubercle small, round, flat, indistinct; thenar tubercle oval, protuberant, double size of palmar tubercle; subarticular tubercles round, conical; supernumerary tubercles round, slightly overlapping to conical, variable in number; webbing absent between fingers ( Fig. 6 View FIGURE 6 A).
Hind limbs slender, moderately long, without calcars or other dermal ornaments; anterior edge of tibia with a row of nearly indistinct tubercles, white; interior tarsal fold indistinct; exterior tarsal fold indistinct; relative length of toes II<III<I<V<IV; discs on toes equal to or slightly smaller than discs on fingers; inner metatarsal tubercle oval, flat; outer metatarsal tubercle absent or indistinct; subarticular tubercles round, conical; supernumerary tubercles round, conical; webbing absent between toes ( Fig. 6 View FIGURE 6 B).
Cloacal opening directed posteroventrally, at upper level of thighs, not ornamented. Skin on dorsal surfaces of head, body and limbs smooth with small white warts scattered irregularly on dorsum, dorsal parts of flanks ( Fig. 5 View FIGURE 5 A) and posterior surfaces of thighs; skin on venter, flanks and ventral surfaces of hind limbs slightly tuberculate.
Coloration: In life, dorsal surfaces of head, body and limbs green ( Fig 1 View FIGURE 1 ); flanks changing from green dorsally to pale brown ventrally through diffuse series of flat white warts; throat and chest greyish brown; belly and ventral surfaces of limbs yellowish brown; irregular white spot, approximately at juncture of each forelimb, in some individuals extending from anterior part of chest to posterior part of throat, bordered or not by smaller white spots; large, round white spots on ventral surface of each thigh proximal to cloaca; ( Fig 2 View FIGURE 2 ); concealed surfaces of hind limbs pink with white bars or spots ( Fig 1 View FIGURE 1 ); inner surfaces of forearm and Fingers II and III pink with white bars; white line on outer edge of forearm, extending to end of Finger IV, serves to separate the dorsal green coloration of the upper surface of the forearm from ventral gray surfaces; a similar tarsal line, not always well defined. The iris is golden with black reticulations.
In preservative, the dorsum is pale blue; the venter is gray, and the belly whitish; concealed surfaces of hind limbs change to grayish pink.
Measurements of Holotype: SVL: 60.2; TL: 28.2; FL: 19.9; HeL: 22; HW: 20; ED: 5.7; TD: 3; IOD: 6; UEW: 6; 1FiL: 8.5; 2FiL: 10; FD: 2; 4TD: 2; InD: 5.
Variation: The pattern on the concealed surfaces of the hind limbs varies from white transverse bars to white spots on a pink background. The subarticular tubercles on the hands and feet are rounded or conical, but they always are protuberant. The shape, number and disposition of the white spots on the chest and ventral surfaces of the thighs also are variable. The variation in measurements is given in Table 1 View TABLE 1 .
Distribution and habitat: In addition to the type locality, one specimen assigned to Phyllomedusa neildi (MHNLS 1503) comes from Curimagua, distrito Petit, in the Sierra de San Luis. Thus, P. n e i l d i is known definitely only from xeric localities on a spur of the Sierra de San Luis, at an elevation of 550 m. The dominant vegetation consists of low trees (to 8 m) called locally “cujíes”, spiny bushes, and cacti, composing a dry semideciduous dwarf forest (bosque muy seco tropical "bmsT" of Holdridge 1967). This species may be endemic to the low, dry lands of the Sierra de San Luis and vicinity, but it also could be more widespread through similar habitats in northwestern Venezuela in the states of Falcón, Lara, Yaracuy and Zulia.
Previously, all known species of what is going to be the Phyllomedusa tarsius group (defined below) were reported to occur from lowland rainforest to cloud forest (bmhT to bmhP of Holdridge 1967), which are the habitats of P. t a r s i u s and P. trinitatis in Venezuela, although the latter can be also found in deciduous forest (bsP). Phyllomedusa venusta is known from rainforest in Darién, Panamá ( Duellman and Trueb, 1967) and northern Colombia to the middle Magdalena Valley, although it inhabits also some localities with a drier climate in Atlántico and Luriza (John D. Lynch, pers. com.). Phyllomedusa coelestis occurs in rainforests in the upper Amazon in Peru and Ecuador.
Natural History: At the type locality, on 22 August 2001, several egg clutches were observed. These were like those described by Kenny (1966) for Phyllomedusa trinitatis in Trinidad. One clutch was encased on the upper surface of a single leaf, about 12 cm wide. Other nests were encased in two or more leaves. Two nests contained 255 and 282 white eggs, surrounded by transparent jelly capsules. Adult males called from bushes at heights of 1.5 m to trees to> 4 m, around ponds. Amplectant pairs were observed in vegetation at various heights above water, but never in the water, as Langone et al. (1985) observed for P. i h e r i n g i. The same night, while in some lagoons many adults were in reproductive activity, in other pools only a few males were calling sporadically. Other species of anurans typical of savannas and xeric habitats of northern South America that were found in the pond where P. neildi was breeding include Chaunus marinus , C. granulosus complex, Dendropsophus microcephalus , D. minutus , Hypsiboas crepitans , Scinax “xsignatus”, Engystomops pustulosus, Pleurodema brachyops, and Leptodactylus insularum .
Vocalization: Three types of notes can be recognized. In the audiospectrogram ( Fig 7 View FIGURE 7 ), each call corresponds to a different individual, but all were recorded at the same time (21:30h) from a single position (air temperature 22°C). The first ( Fig 7 View FIGURE 7 A) consist of a single note of 104 ms of length and 707 Hz of dominant frequency (fundamental frequency of 168 Hz). The second ( Fig 7 View FIGURE 7 B) is a series of eight notes of 871 ms, with a dominant frequency of 736 Hz (fundamental 140 Hz). The third ( Fig 7 View FIGURE 7 C) was the most common (that night) and consists of two notes, one principal with a duration of 164 ms and a dominant frequency of 843 Hz, (fundamental 252 Hz), and a secondary of 104 ms. The length of the complete sequence is 302 ms. Rivero & Esteves (1969) showed an audioespectrogram of the call of Phyllomedusa trinitatis , but they did not described it. In their spectrogram, there is a principal note, followed by five secondary notes; as can be extrapolated, the dominant frequency is approximately at 800 Hz, while the fundamental is at 500 Hz; the duration of the sequence is of 1.1 sec. Phyllomedusa neildi can emit up to fifteen consecutive secondary notes. Kenny (1966) described a distress call for P. trinitatis that was not heard in P. n e i l d i. Calls of the Phyllomedusa tarsius group species have been never well analyzed. We cannot conclude any important difference among them. The call of P. c a m b a (out of the P. t a r s i u s group in this paper) has also a dominant frequency of 860 Hz.
TABLE 1. Measurements (in mm) of the type series of Phyllomedusa neildi sp. nov. SD (= Standard Deviation).
| Males (n = 6) | Females (n = 2) | |||
|---|---|---|---|---|
| Range | Mean SD | Range | Mean SD | |
| SVL | 55.263.8 | 59.8 3.58 | 70.576 | 73.2 3.89 |
| TL | 2630 | 27.8 1.55 | 3234 | 33 1.41 |
| FeL | 16.820.5 | 19.2 1.35 | 22.622.8 | 22.7 0.14 |
| HeL | 1922 | 20.7 1.11 | 23.624 | 23.8 0.28 |
| HW | 18.820.6 | 19.6 0.70 | 24.224 | 24.1 0.14 |
| ED | 55.9 | 5.4 0.43 | 5.97.8 | 6.8 1.34 |
| TD | 33.4 | 3.1 0.16 | 3.84 | 3.9 0.14 |
| IOD | 5.87 | 6.4 0.59 | 8 | 8 0 |
| UEW | 56.1 | 5.6 0.42 | 66.2 | 6.1 0.14 |
| 1FiL | 6.59 | 7.6 1.16 | 8.510 | 9.2 1.06 |
| 2FiL | 7.210.1 | 8.9 1.05 | 10.211.6 | 10.9 0.99 |
| FD | 1.82 | 1.9 0.08 | 22.5 | 2.2 0.35 |
| 4TD | 1.32 | 1.8 0.27 | 22.2 | 2.1 0.14 |
| InD | 45 | 4.6 0.37 | 5.55.5 | 5.5 0 |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.