Miotragocerus Stromer, 1928
|
publication ID |
https://doi.org/10.5281/zenodo.4650779 |
|
persistent identifier |
https://treatment.plazi.org/id/9636CE11-B47A-FFB4-FD27-D8E8FE5FFD3E |
|
treatment provided by |
Felipe |
|
scientific name |
Miotragocerus Stromer, 1928 |
| status |
|
Genus Miotragocerus Stromer, 1928
Tragocerus Gaudry, 1861: 393 View in CoL ( pars).
Miotragocerus Stromer, 1928: 36 .
Graecoryx Pilgrim F Hopwood, 1928: 54 ( type species: G. vallenciennesi ( Gaudry, 1861)) .
Sivaceros Pilgrim, 1937: 792 ( type species: S. gradiens Pilgrim, 1937 ).
Pikermicerus Kretzoi, 1941: 342 ( type species: P. gaudryi Kretzoi, 1941 ).
Dystychoceras Kretzoi, 1941: 336 ( type species: D. pannoniae Kretzoi, 1941 ).
NOMENCLATURAL REMARKS. — Most of the late Miocene boselaphines from the eastern Mediterranean region originally referred to the genus Tragocerus Gaudry, 1861 View in CoL ( Gaudry 1862 -1867, 1873; Schlosser 1904; Pavlow 1913; Borissiak 1914; Mecquenem 1924; Andree 1926; Pilgrim F Hopwood 1928) have been lately transposed either to Miotragocerus Stromer, 1928 or to Tragoportax Pilgrim, 1937 . The main differences between these two genera concern the horncore pattern, some cranial and dental features and the presence of horns in females. Nevertheless, opinions among authors vary significantly and thus several species appear to interchange between the two genera (see discussions in Kretzoi 1968; Gentry 1971; Thomas 1979; Solounias 1981; Moyà-Solà 1983; Bouvrain F Bonis 1984; Bouvrain 1988, 1994b; Spassov F Geraads 2004; Bouvrain F Heintz pers. comm.). A long debate also took place about the validity of Graecoryx Pilgrim F Hopwood, 1928 , based on Tragocerus valenciennesi Gaudry, 1861 from Pikermi. Several authors consider this taxon artificial, founded upon material of young and female individual of other related genera ( Bohlin 1935; Bouvrain 1988; Spassov F Geraads 2004), while others allot a true value to the genus (Moyà-Solà 1983; Köhler 1987; Romaggi 1987). Avoiding long repetitions of the systematic history of all these taxa, I shall focus on the most recent options found in the literature, trying to eliminate distortion on nomenclature.
Moyà-Solà (1983) placed Dystychoceras pannoniae Kretzoi, 1941 from Sopron, Hungary, in Miotragocerus and restored Graecoryx for G. valenciennesi . Romaggi (1987) followed Moyà-Solà (1983) about Miotragocerus but rejected Tragoportax and placed all the classical European tragoceres under Graecoryx . Furthermore, he disagreed with the synonymy of Graecoryx with Miotragocerus putting the argument of the different premolar/molar pattern (more advanced in Miotragocerus according to this author). My observations do not support his conclusion; the observed variability in the dental structure of these taxa cannot be considered as exceeding that of forms belonging to the same genus, and indeed, it is significantly smaller than the differences observed between species included by the author in Graecoryx .
In a more recent review, Bouvrain (2001) and Bouvrain F Heintz (pers. comm.) propose to restrict the name Miotragocerus only to the type species M. monacensis Stromer, 1928 and – on the basis of horned females – to place the rest of the related species ( D. pannoniae , P. gaudryi , T. leskewitschi ( Borissiak, 1914) and probably S. gradiens ) in Dystychoceras Kretzoi, 1941 because Miotragocerus seems to them insufficiently established. In my opinion, the basic skull and horn-core characters that can be extracted from Stromer’s specimen are present in D. pannoniae and they seem sufficient to both define these two forms at generic level and distinguish them from other genera (see also Moyà-Solà 1983; Romaggi 1987). As Kretzoi (1941: 338) sets out, the Sopron frontlet presents great similarity with Sivaceros Pilgrim, 1937 and extending his thought, it is evident that the differences between the type species of Dystychoceras and Mioragocerus do not exceed those observed between S. gradiens and S. vedicus Pilgrim, 1939 . Actually, Sivaceros appears close to Miotragocerus and Graecoryx and a synonymy is possible, as it is already suggested by other authors. Phronetragus Gabuniya, 1955 ( type species P. arknetensis ; Meladze 1967) also shows a great number of common characters with both Miotragocerus and Graecoryx and its distinction at genus level does not seem to me justifiable but final conclusion presupposes the study of the original material.
Spassov F Geraads (2004) revise both Tragoportax and Miotragocerus . Although I do not agree with all the interpretations, especially at subgeneric and species level, and the consequent phylogenetic relationships, I have no substantial objections to the Miotragocerus concept provided by the authors but I certainly regard Pikermicerus as a junior synonym of Graecoryx since the type species of both genera are based on material representing the same taxon in the Pikermi fauna (see below). Hitherto, the synonymy status between Miotragocerus and Graecoryx has been unclear since both genera were founded the same year. Gentry (1971: 234, footnote) wrote that the copy of Pilgrim F Hopwood’s book was accessioned in the Palaeontology Library of the British Museum (Natural History) at a date close to 28 June 1928. Ms Polly Smith, Assistant Archivist, and Ms Susan Snell, Archives Manager of the Natural History Museum, London, informed me (pers. comm. 2002, 2003) that the book is first mentioned in the Publications Presentations Books (ref. DF 508/5) on Saturday 23 June 1928. On that day the book was read and discussed in the Standing Committee of the British Museum (Natural History), confirming its price and a copy was set before the Museum Trustees. Hence, it seems that the book would not have been made available to members of the public outside the Museum before Monday 25 June 1928 at the earliest. Anthea Gentry (pers. comm. 2002) suggests that since the date of publication of a book is when copies are first distributed, the 23 June 1928 could look like a first distribution and therefore the date of publication. But it is more likely that the committee meeting constituted the formal last stage of pre-publication vetting and, indeed, seems to have been the occasion on which the selling price of the book was decided. According to the Publication Sales register of that peri- od (Ms S. Snell, pers. comm. 2003) the first sales of Pilgrim F Hopwood’s book to two firms of retail booksellers, Oxford Press and Bernard Quaritch were only registered on 3 July 1928 (ref. DF 500/5). In this case it seems logical to accept as first publication date, a date in-between 25 and 29 June. This date clearly postdates that of Stromer’s monograph, given as 5 May 1928 on the front cover of the journal and therefore, Miotragocerus does have priority over Graecoryx .
Miotragocerus valenciennesi ( Gaudry, 1861) Tragocerus valenciennesi Gaudry, 1861: 393 , pl. VIII, figs 4, 5; 1862-1867: 288, pl. XLVIII, figs 2, 3.
Graecoryx valenciennesi – Pilgrim F Hopwood 1928: 55, pl. VIII, fig. 2, pl. IX, figs 4, 5. — Moyà-Solà 1983: 103. — Romaggi 1987: 277.
Pikermicerus gaudryi Kretzoi, 1941: 342 , fig. 2.
Miotragocerus monacensis – Solounias 1981: 102, fig. 26.
Miotragocerus valenciennesi – Solounias 1981: 105.
Tragoportax gaudryi – Moyà-Solà 1983: 124.
Dystychoceras gaudryi – Bouvrain 2001: 238.
Miotragocerus ( Pikermicerus) gaudryi – Spassov F Geraads 2004: 353.
NOMENCLATURAL REMARKS. — The present situation does not resolve the issue of how many boselaphine species exist in the Pikermi fauna. In addition to Tragoportax amalthea (Roth F Wagner, 1854) , two more species have been described: Graecoryx valenciennesi and Tragoportax gaudryi . The last species is admitted into the Pikermian sample by Moyà-Solà (1983) on the basis of Pikermicerus gaudryi Kretzoi, 1941 (also referred to as Dystychoceras gaudryi or Miotragocerus gaudryi ). The dental material of Pikermi does not support, however, a third species in the locality. The frontlet BMNH M12992 ascribed to Graecoryx valenciennesi by Pilgrim F Hopwood (1928) most probably belongs to a young male individual (age classes xI-II, see below) of a form with horn-core pattern that should allow (through the morphotypes BMNH M11423 View Materials a and M32170 View Materials ) to a mature morphology similar to that of T. gaudryi (BMNH M11423 View Materials b; MNHN PIK 2366, PIK 2448). The data from Sebastopol ( Borissiak 1914), Halmyropotamos ( Melentis 1967), Hoewenegg ( Romaggi 1987) and Akkaşdagwı fully support such a conclusion. The type specimen of Graecoryx valenciennesi (MNHN PIK 2367) and the skull specimen BMNH M11430 View Materials are attributed to the same species by Pilgrim F Hopwood (1928); the last specimen is erroneously referred to as hornless by Solounias (1981: 107) and figured later as T. gaudryi by Moyà-Solà (1983: pl. 17, fig. 1). Actually, both specimens belong to horned females, whose morphology strongly resembles the boselaphine females from Nikiti-1 (Kostopoulos F Koufos 1996), Piera (Moyà- Solà 1983), Dytiko ( Bouvrain 1988), Hoewenegg ( Romaggi 1987), Pikermi ( Roussiakis 1996) and Akkaşdagwı (see below), and making due allowances, they should be regarded as conspecific with PIK 2366 ( type specimen of Pikermicerus gaudryi ). Moreover, the dentition of BMNH M11430 View Materials is indistinguishable from those referred to T. gaudryi . Thus, Graecoryx valenciennesi of Pilgrim F Hopwood (1928) and Tragoportax gaudryi of Moyà-Solà (1983) from Pikermi are the same species (see also Romaggi 1987). From this point of view, the name valenciennesi is preferable to gaudryi because the principle of priority should be applied even if “two or more generations, forms, stages, or sexes of a species are named as different nominal taxa” ( ICZN 1999: Art. 23.3.2.2). Hence, we should recognize Miotragocerus valenciennesi ( Gaudry, 1861) as the second boselaphine of Pikermi, in contrast to previous suggestions (e.g., Spassov F Geraads 2004: 360 and literature listed herein).
MATERIAL EXAMINED. — Skull: AK5-597; part of skull: AK11-64 (a, b, c); left horn-core: AK14-14; P2- M3: AK5-605dex ( LPM = 92.3, LP = 42.0, LM = 49.8), AK12-75sin ( LPM = 95.5, LP = 44.4, LM = 53.9); p2-m3: AK2-322dex (Lpm = 98.6, Lp = 42.5, Lm = 56.3), GOK-196sin (Lpm = 102.0, Lp = 47.9, Lm = 55.0).
DESCRIPTION
Skull
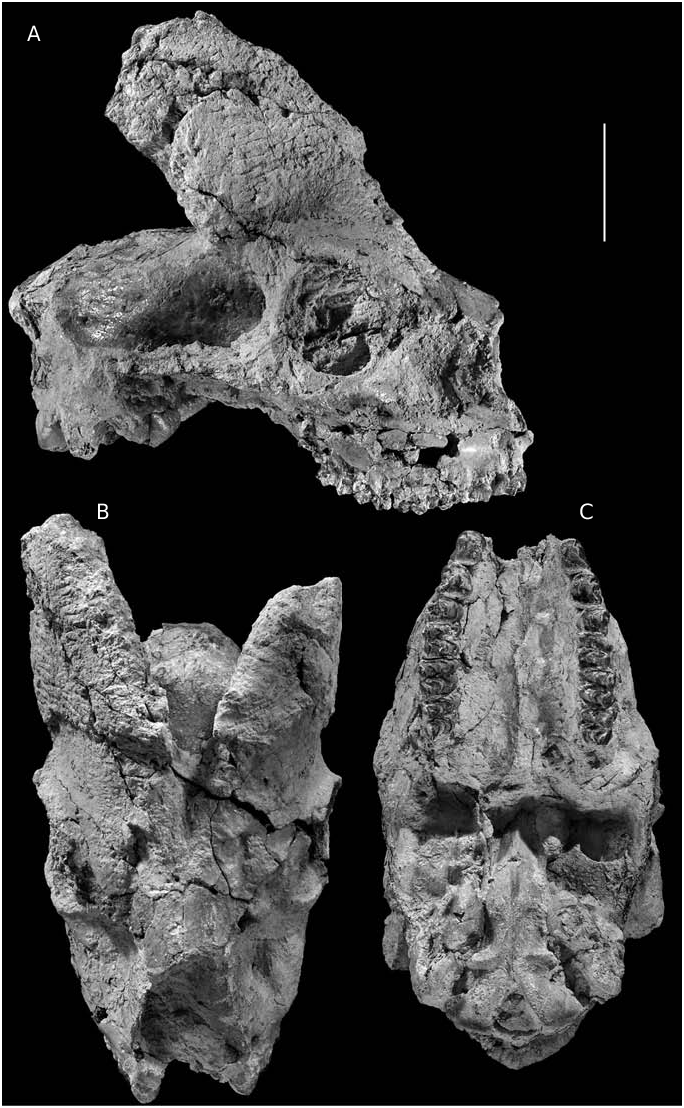
The skull specimen AK5-597 is beautifully preserved, except for the anterior part of the muzzle (nasals, premaxillae) that is missing ( Fig. 12 View FIG ; Appendix: Table 10). Judging from the horncores and dentition it belongs to an adult male. Retaining the palate horizontal, the cranial roof forms an angle of about 120° with the face. The frontals are strong and clearly elevated between the horn-cores. The fronto-nasal suture forms a reverse “U”. Ahead of the horn-cores the frontals form a wide furrow towards the nasals; its lateral margins consist of the frontal prolongation of the horn-core’s keels. The sub-trianguar supraorbital foramens are not sunken into pits. The inter- and opisthofrontal area of the cranial roof is rough, forming a wide rugose area. Just behind the horncores the frontals are slightly depressed. There are no postcornual grooves. The narial notch seems to be large; its posterior end is placed above P3. The infraorbital foramen is large, situated above P2- P3. The lachrymal bone is wide and probably touches the premaxillar. A relatively long and narrow ethmoidal fissure opens between the frontal, nasal and lacrymal bones. The lacrymal fossa (“larmier” according to Gaudry) is placed just in front of the upper half of the orbit ( Fig. 12A View FIG ). It is elliptical-rounded in shape, deep and well delimit- ed (20 × 15 mm with vertical greater axis). The orbit is slightly projected laterally, small and well rounded. Its anterior end is placed above the middle of M2. The facial crest is well developed, blunt and bulge, limited above P3-M3. Above P4 and in front of the orbit appears a deep oval fossa with well developed posterior border (preorbital fossa). The zygo-temporal arch runs parallel to the braincase and the temporal ridges are strongly developed. The interparietal is well developed and rhomboid shaped ending posteriorly in a well developed supraoccipital. The braincase is narrow and relatively long. The occiput is pentagonal shaped concave in lateral view and with strong and sharp nuchal crest. The strong paroccipital processes project below the lower level of the condyles, and in lateral view they are placed slightly in front of them. The condyles are small. The basioccipital axis is parallel to the cranial roof and the basioccipital-palatal angle very obtuse. The posterior tuberosities of the basioccipital are strong, elongated crest-like and vertical to the sagittal plane ( Fig. 12C View FIG ). The anterior tuberosities are smaller, oval shaped and slightly swelling. A single ridge runs along the basioccipital axis between the anterior and posterior tuberosities but it is replaced by a narrow groove in front of the anterior tuberosities. The small oval foramen is placed well in front of the anterior tuberosities. The auditory bulla is small, elliptical and with major axis parallel to the sagittal plane. The palate is wide and flat. The median indent at back of the palate opens anteriorly to the lateral ones and well behind M3 ( Fig. 12C View FIG ). The pedicle is short, especially in the posterior part and its contact with the horn-core is well marked. The horn-cores set close together anteriorly at the base and tilt moderately backwards ( Fig. 12A, B View FIG ). Their anterior end is situated above the middle of the orbit and their posterior face above the middle of the temporal fossa. In frontal view the divergence angle of the horn-cores is about 50°. Their cross-section is weakly sub-triangular at the base becoming rapidly elliptical and strongly compressed laterally. The postero-internal dihedre is not strongly developed but the level of maximum transverse width lies posteriorly. A smooth anterior keel is present. The anteroposterior axis of the horn-core base forms an angle of 40° with the sagittal plane. The anterolateral surface of the horn-core bases bears strong exostoses. The lateral face is slightly convex and the median one almost flat.
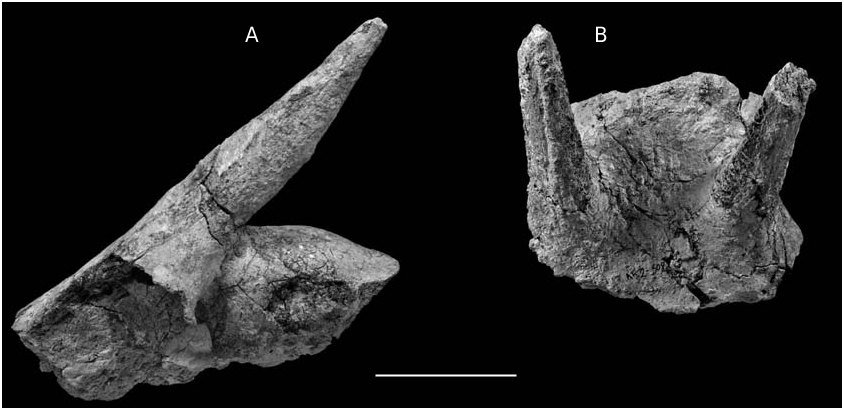
Young individuals (AK11-64, AK14-14)
The skull AK11-64 ( Fig. 13A View FIG ) preserves the frontal and part of the parietal region as well as both horn-cores. The face slopes smoothly on the cranial roof. The distance between the supraorbital pits, situated far below the horn-core bases, is 33.5 mm. The interfrontal and frontoparietal sutures are open. The frontals are not elevated between the horns. The length from the frontonasal suture to the nuchal crest is 140 mm and the length of the mid-frontals is about 88 mm. The orbits are strongly projected laterally. The pedicles are long and bear sinuses. There are no postcornual grooves. The horn-cores are distant at the base and almost parallel between them. They are inserted above the posterior part of the orbits obliquely to the sagittal plane and strongly inclined backwards in side view. From the base to the top they present a slight reverse torsion (clockwise). The horn-core cross-section is elliptical with traces of a blunt anterior keel. Their dimensions are: DTb = 21 mm; DAPb = 30.3 mm and their length at about 90 mm. In lateral view the posterior face of the horn-core appears convex, whereas the anterior one from convex at the base becomes concave in the upper half, following a relatively abrupt reduction of the horn-core dimensions towards the apex. A similar morphology is observed on a left horncore AK14-14 ( Fig. 14 View FIG ), which, however, is larger (DT b = 31 mm, DAP b = 45.4 mm; L> 80 mm), significantly heavier (more compact internal texture) and with swelling basal part.
Females (frontlet AK2-502)
The horn-cores are almost straight, without keels, inserted above the posterior part of the orbits, strongly sloping backwards (40° with the cranial roof) and slightly diverged ( Fig. 13B View FIG ). They are far apart at their bases and strongly compressed medio-laterally, especially in their upper parts (DT b = 22.6dex and 22.1sin; DAP b = 32.4dex and 32.0sin). Their internal surface is almost flat, while the lateral one is smoothly convex. The contact with the pedicles is not well marked. The frontals are not elevated and there is not postcornual groove. The width of the skull just behind the horn-cores is about 76 mm. The general morphology of this frontlet clearly reminds that of female individuals of late Miocene miotragoceres described from Spain, Germany, France and Greece (Moyà-Solà 1983; Romaggi 1987; Bouvrain 1988; Kostopoulos F
Bovidae (Artiodactyla) from Akka s daw gı
Koufos 1996; pers. obs.). Therefore, I provisionally assign the specimen AK2-502 to a female individual of the previously described form.
Dentition
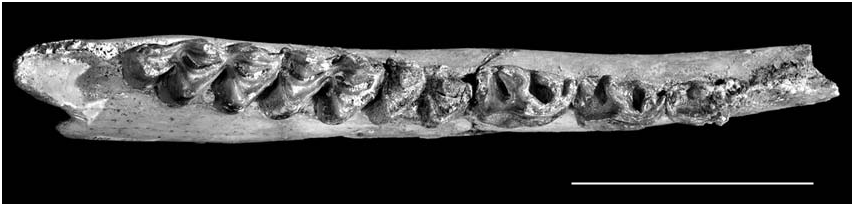
The mandibular ramus (GOK-196) is shallow (height between p4-m1 = 27 mm; in front of p2 = 19.3 mm). The mental foramen opens 32.5 mm in front of p2.
The teeth are brachyodont with rough enamel and strong upper and long-narrow lower premolars ( Fig. 15 View FIG ). The premolar/molar ratio varies between 81 and 85 for the upper toothrow and from 75 to 87 for the lower one. P2 is as long as P3 while P4 is clearly shorter. The P2 is bilobed with strongly developed paraconeparastyle-protocone complex. The P3 is quadrangular and less molarized than P2. Among the molars, the M2 predominates because of its larger size. The labial styles and ribs are well developed but not strongly projected. A small central islet is occasionally present in M1,2 but is usually missing on M3, where a basal cingulum is present. A vestigial basal pillar is also occasionally present. Lingually, the lower premolars are slightly molarized. The paraconid of p2 is very weak. The p3 has stronger paraconid than parastylid, situated vertically to the anteroposterior axis of the tooth but projected posteriorly towards the base. The metaconid of p3 is free, elongated and backwardly directed. The entoconid is sub-triangular and stronger than the entoconid. The p4 is similar to p3 but with stronger paraconid, forming sometimes a posterior vane that tends to touch the metaconid ( Fig. 15 View FIG ). The latter one is extended anteroposteriorly. The entoconid of p4 is strong, fused rapidly with the endostylid. The lower molars bear a weak anterior fold and a small basal pillar that increases from m1 to m3. On the lingual face there is also a thin basal pillar. The talonid of m3 is single-tubercled.
BOSELAPHINE HORN DEVELOPMENT
Several palaeobiologists discussed the changes of boselaphine horn-cores due to ontogenetic growth and their effects on the taxonomy of the tribe ( Bohlin 1935; Thenius 1948; Thomas 1979, 1984; Solounias 1981, 1990; Janis F Scott 1987; Bouvrain 1988). Trying to evaluate this morphological plasticity in relation to the Akkaşdagwı specimens, I examined the skull collection of extant Boselaphus tragocamelus (Pallas, 1766) stored in the Natural History Museum of London. Since females of this species are hornless, I have chosen a set of 15 male skulls bearing toothrows, as the basis for my observations. My results, divided in four age classes, are as follows:
– Class I: ≈ 1-2 years old (M3 still within the alveoli, M2 just erupted, D2-D4 weakly-moderately worn): the pedicle directs posterolaterally and it is well developed, cylindrical, and longer anteriorly than posteriorly. The horn-core inserts behind the orbit, placed normally above the pedicle, and directs backwards and slightly upwards. It is short and slender and in side view shows a slightly concave upper (anterior) and slightly convex lower (posterior) surface; the cross-section is regularly rounded to oval. In its proximal part the horn-core surface bears shallow discontinuous longitudinal grooves, while distally it is more porous. The temporal ridges are well developed but they do not raise like a ridge and they do not touch each other distally. The frontals are smooth, non elevated, and there is no rugose area.
– Class II: ≈ 3 years old (P2-P4 unworn, D4 worn, M3 just erupted): the horn-cores are identical with Class I but slightly larger, certainly longer and directed more upwards. The frontals form two elongated bulges between the pedicles and the supraorbital pits. There is no rugose area on the frontoparietal region, but the temporal ridges are stronger, ridge-like and touch each other posteriorly.
– Class III: ≈ 4-5 years old (full permanent dentition in first stage of wear): the frontals are slightly raised between the horn-cores forming a distinct broad intercornual plateau. The horn-core stops lengthening. Significant amount of new bone has been added above the frontal bulges and around the pedicle, enlarging and prolonging anteriorly the horn-core base. The appositional process in the anterobasal part also allows to the formation of a strong anterior keel restricted in the lower third. A postero-internal dihedre develops and the cross-section becomes triangular at the basal half. The temporal ridges are very strong and a rugose area appears on the frontoparietal region (behind and between the horn-cores).
– Class IV:> 6 years old [full permanent dentition in advanced stage of wears: the rugose area extends above the orbits and both the intercornual plateau and the fronto-parietal depression become stronger. The horn-core is similar with that of age class III but slightly thicker anterobasally and showing several deep longitudinal furrows. The contact between the terminal end of the anterior keel and the upper unkeeled part of the horn-core forms a step, while another step is formed close to the base. The sheath presents two clear demarcations in its anterobasal part reflecting the internal steps. The length difference between sheathed and unsheathed horn at this stage is small (≈ 15%)
Evidently, the horn-core and frontal morphology of the specimen AK11-64 fits pretty well with that of a Boselaphus Blainville, 1816 in age Class I. The horn-core AK14-14 cannot be placed exactly in this scheme but, relatively speaking, it could correspond to a stage between age Classes II and III. Both Janis F Scott (1987) and Solounias (1990) discuss the issue of the horn development in Bovidae and especially in Boselaphini , concluding, however, in somewhat conflicting results. My observations show that the early development of horns in Boselaphus does not differ structurally from that of other Bovidae . Horned females of late Miocene boselaphines seem also to follow the normal process described by Janis F Scott (1987: 10-14). Moreover, the horn-cores (and consequently the sheath) of juvenile Boselaphus clearly resemble those of adult living Tetracerus Leach, 1825 , early-middle Miocene Eotragus Pilgrim, 1939 and middle Miocene Strepsiportax Pilgrim, 1937 and they appear to be similar to the morphology observed in female and young male individuals of late Miocene boselaphines (e.g., AK11-64, AK2- 502). Hence, I will agree with Janis F Scotts’ statement (1987: 14) that there is no reason to believe that the horn growth-mechanism of extant and extinct boselaphines is any different from other bovids, and, as a result, I should refuse the hypothesis of Solounias (1990: 435) for a boselaphine taxonomic distinction based on a bovid biphyly.
In contrast to the horn growth-mechanism of boselaphines proposed by Bohlin (1935) and Solounias (1981, 1990), my data show that until age Class II the main developmental process of Boselaphus horns is the lengthening. Somewhere between age Classes II and III lengthening is slowing significantly down, the sheath extends downwards covering the pedicle and the backend of the pre-formed frontal bulges and thus preosteous substance (see Janis F Scott 1987) expands anterobasally, adding appositionally new bone around the base. From age Class III and then after horn growth occurs mainly around the base with a progressive extension of the described mechanism anterobasally as the horn increases slowly in length. Depending on the rates of horncore lengthening (which in turns may reflect ecological factors), the process produces successive steps along the keeled anterior face, which is a result of the dihedral apposition of bone on the frontal bulges. The anterior horn-core steps are expressed as demarcations on the anterior sheath surface.
Although particular, the described mechanism is not unique among Bovidae and analogies can be detected in the horn development of ovibovines ( Allen 1913). The described procedure seems also to explain pretty well the horn-core morphology of late Miocene boselaphines, given that different rates (or stability) in length development between the early and late ontogenetic stages in combination with a continuous forward growth could provide different horn-core shapes in distinct taxa. Solounias (1990) is right, however, to suggest relations between the horn base of the late Miocene boselaphines and the protrusion fields above the frontal bulges of Boselaphus . But to my viewpoint, these fields are the rudiments of an appositional process that occur(ed) later in the ontogenetic development of horns and which seems to have been degenerate in Boselaphus . The main difference between Boselaphus and late Miocene boselaphines is that the frontal bulges of the earlier forms are entirely covered by horn bone, whereas in the living form the process is incomplete, leaving a long rough area between the horn base and the supraorbital pits.
As Janis F Scott (1987) point out, the hypothesis of multi-tined Miotragocerus sheath is unlikely to be correct. Based on Boselaphus , I think, however, quite possible that the external horn morphology of Miocene boselaphines could follow the internal one.
COMPARISON
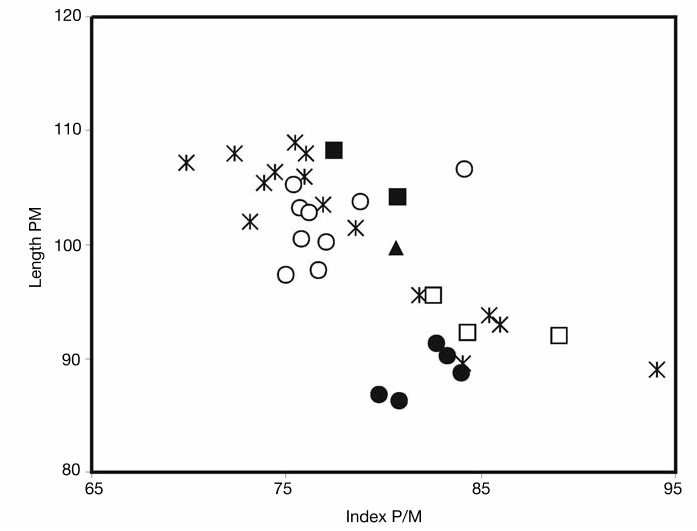
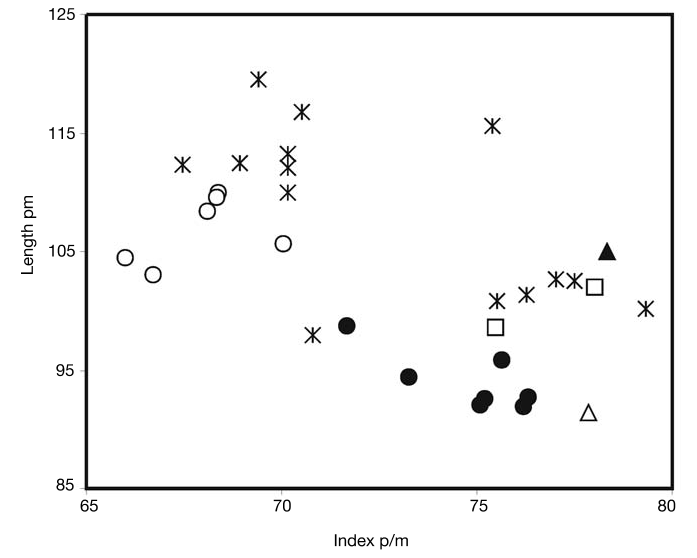
Hitherto boselaphines have been scarcely recognized in the late Miocene of Turkey (see Köhler 1987; Gentry 2003). The Akkaşdagwı form shows clear affinities with Miotragocerus (horned females, backwardly inclined and untwisted male horncores smoothly keeled with an anterior demarcation, high premolar/molar ratio) and especially with the species M. valenciennesi from Pikermi. The skull AK5-597 is almost identical to the specimens MNHN PIK 2366 and PIK 2448 sharing in common a similar sloping of the face on the cranial roof, a relatively short opisthocranium, a small and well defined lacrymal fossa placed in the anterosuperior part of the orbit, a relatively deep preorbital fossa limited posteriorly above M1-P4, an infraorbital foramen placed above P2, and a small auditory bulla, placed just in front of the strong posterior tuberosities of the basioccipital. The dental proportions of the Akkaşdagwı form fall within the range of the Pikermian sample ( Figs 16 View FIG ; 17 View FIG ), being clearly distinct from those of Tragoportax amalthea (Roth F Wagner, 1854) and T. rugosifrons ( Schlosser, 1904) . It is therefore no doubt for the inclusion of the Akkaşdagwı boselaphin in Miotragocerus valenciennesi . The species is also present in the whole fauna of Samos (AMNH 22766 from Q6, AMNH 86556 and 86557 from Q2, AMNH 20572 from Q5, PIM 65 and AeMNH MTLA11). Melentis (1967) described from the middle Turolian locality of Halmyropotamos, Greece, several races of Tragoportax amalthea . Roussiakis (1996) recognizes in this material several specimens that could be attributed to “ Tragoportax gaudryi ” ( Melentis 1967: pls XV, XVI, figs 2, 3, pl. XVII, fig. 2) and in agreement with him I refer this material to Miotragocerus valenciennesi . The adult skull from Achladi (Euboea, Greece) figured by Mitsopoulos (1947: fig. 3) as “ Tragocerus ” amalthea is also ascribed with some confidence to Miotragocerus valenciennesi .
Moyà-Solà (1983) and Bouvrain (1988) followed by Spassov F Geraads (2004) recognize several subspecies of M. valenciennesi to which Bouvrain F Heintz (pers. comm.) impute lately specific value. This makes that Miotragocerus is a highly diversified genus. Except for M. monacensis and M. pannoniae from the Vallesian of Central Europe and M. valenciennesi from the middle Turolian of E Europe, M. crusafonti is known from the early Turolian of the Iberian Peninsula and M. macedoniensis from the late Turolian of Greece. The unpublished Miotragocerus (= Graecoryx ) “ andancensis ” ( Romaggi 1987) from France appears to be very similar to M. crusafonti from Piera both on the male and female morphology, and I suggest that they could be regarded as synonyms. These two forms from the early Turolian of Western Europe present some interestingly distinctive characters from the eastern ones (such as the reduced size and details of female skull morphology) that merit a more careful approach. The form from Piera ( Spain) shows a stronger cranio-facial angle, a more inflated braincase and a certainly smaller size than the Pikermi and Akkaşdagwı Miotragocerus . The attribution of the Mont Lubéron form to M. valenciennesi (Moyà-Solà 1983) seems quite doubtful and Romaggi (1987) considers this form as belonging to Tragoportax amalthea .
Miotragocerus macedoniensis Bouvrain, 1988 from Dytiko is different from the Akkaşdagwı form in having a generally smaller size, smaller and probably longer horn-cores, un-concave occiput vertical to the cranial roof, larger auditory bulla, shorter basioccipital and slightly smaller premolar/molar ratio associated with a clearly smaller toothrow ( Figs 16 View FIG ; 17 View FIG ). Spassov F Geraads (2004: 341, 356) throw doubt upon the generic affinities of the Dytiko form and prefer to transfer it to Tragoportax because of “the shape of the basioccipital and the presence of a fronto-parietal post-cornual depression”. I believe, however, that most of the characters ascribed by them to the Dytiko form (Spassov F Geraads 2004: table 5) are rather misinterpreted: the basioccipital of the Dytiko form shows a strong median crest which is not placed into a groove formed by paired broadly blunt ridges between the anterior and posterior tuberosities on each side; the occiput narrows relatively abruptly towards its top; the temporal lines are moderately developed; the intercornual region is definitely narrow and not raised; the horn-core divergence is certainly weaker than in Tragoportax and the horns run rather parallel to each other; the postero-internal dihedral is not developed; the premolar row is certainly longer comparatively to Tragoportax (compare values in Bouvrain 1988, 1994b; Spassov F Geraads 2004) and the p3, p4 are positively Miotragocerus -like. In my opinion, this set of characters lets little doubt about the attribution of the species to Miotragocerus . The interpretation of the post-cornual depression is more delicate: the intenseness of this character is a relation of how much the intercornual plateau is raised and how strongly the temporal ridges appear. Both features are not exaggerated in the Dytiko boselaphin and they do not significantly differ from those observed in other specimens ascribed to Miotragocerus (such as AK5-597 from Akkaşdagwı, I-122 from Sebastopol, NKT-220 from Nikiti-1, U-57 from Höwenegg, PIK 2366 from Pikermi, AMNH 86556 from Samos and HD-2010 from Hadjidimovo). Nevertheless, the development of rugosities is quite stronger in Dytiko than in M. valenciennesi .
Among the material of Tragocerus leskewitschi Borissiak, 1914 from Sebastopol, there is a part of skull ( Borissiak 1914: pl. IV, fig. 5) with clear affinities to that of Miotragocerus . Borissiak (1914) regards the horn-core pattern of this specimen as a different morphotype than that of the type I-122, but a cast of the latter specimen stored in BMNH (M15761) shows that the horn-cores are badly attached to the skull: a relatively thick zone of plaster interposes between the pedicles and the preserved part of the horn-cores. Bouvrain (1994b), Spassov F Geraads (2004) and Bouvrain F Heintz (pers. comm.) suggest a provisional generic similarity of this Vallesian species with Miotragocerus / Dystychoceras and I fall in with them, maintaining however Miotragocerus . Miotragocerus leskewitschi also presents interesting similarities with Sivaceros vedicus (which is however based on a relatively immature skull), as well as to Graecoryx bonus Korotkevich, 1981 from Belka. In comparison with the Akkaşdagwı form, M. leskewitschi differs in the less inclined face, simpler and smaller P2, more posteriorly placed orbit and more inclined horn-cores.
The Nikiti-1 boselaphin is originally ascribed to Tragoportax gaudryi by Kostopoulos F Koufos (1996) and referred to as Dystychoceras sp. in Bouvrain (2001) but seems to be closer to the Hungarian species M. pannoniae . The male skull NKT-220 is almost identical to the male morphology of the latter species (according to the figures and descriptions of Romaggi 1987), while the female skull NKT-120 slightly differs in the longer opisthocranium and the curved horn-cores. I suggest therefore referring the NKT form to Miotragocerus cf. pannoniae . Nikiti-1 boselaphin differs from the Akkaşdagwı form in its smaller size, the shorter opisthocranium, the less developed rugose region and temporal ridges and the un-concave occiput.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Miotragocerus Stromer, 1928
| Kostopoulos, Dimitris S. 2005 |
Dystychoceras gaudryi
| BOUVRAIN G. 2001: 238 |
Graecoryx valenciennesi
| ROMAGGI J. - P. 1987: 277 |
Miotragocerus monacensis
| SOLOUNIAS N. 1981: 102 |
Miotragocerus valenciennesi
| SOLOUNIAS N. 1981: 105 |
Pikermicerus
| KRETZOI M. 1941: 342 |
Dystychoceras
| KRETZOI M. 1941: 336 |
Pikermicerus gaudryi
| KRETZOI M. 1941: 342 |
Sivaceros
| PILGRIM G. E. 1937: 792 |
Miotragocerus
| STROMER E. 1928: 36 |
Tragocerus
| GAUDRY A. 1861: 393 |
Miotragocerus valenciennesi (
| GAUDRY A. 1861: 393 |