Flabesymbios commensalis (Moore, 1909), 2012
|
publication ID |
https://doi.org/10.11646/zootaxa.3203.1.1 |
|
persistent identifier |
https://treatment.plazi.org/id/8C476837-FFD6-FFD4-FF79-FF46FAE5F8C1 |
|
treatment provided by |
Felipe |
|
scientific name |
Flabesymbios commensalis (Moore, 1909) |
| status |
comb. nov. |
Flabesymbios commensalis (Moore, 1909) View in CoL n. comb.
Figures 22 View FIGURE 22 , 23 View FIGURE 23
Flabelligera commensalis Moore 1909a:286–288 View in CoL , Pl. 9, Figs. 62–63; Günther 1912:16, Hartman 1961:117–118, frontispiece ( partim); Stasek 1966:11–12; Light 1978:682–683 ( partim); Loi 1980:137; Blake 2000:10, Fig. 1.3 View FIGURE 1 ( partim).
Flabelligera haerens Chamberlin 1919a:16 View in CoL ; Hartman 1938:14.
Flabelliderma commensalis: Hartman 1969:285–286 View in CoL , 3 Figs. ( partim).
Type material. Northeastern Pacific. Holotype of Flabelligera commensalis ( CAS-20706 ), and four paratypes (three as cotypes: ANSP-2532 ; and one paratype: CAS-20707 ), Picnic Tables (= Pescadero Point, after Stasek 1966), near Hopkins Marine Station, Monterey , California, among the spines of Strongylocentrotus purpuratus , 14 Aug. 1904, M. H. Spaulding, coll. Holotype of Flabelligera haerens ( MCZ-2163 ), kelp holdfast, Laguna Beach , California, 12 Aug. 1917, W.A. Hilton coll. (dried-out) .
Additional material. Northeastern Pacific Ocean. On S. purpuratus: Two specimens ( LACM-AHF-2576 ), Bird Rock, La Jolla, Calif. , 30 May 1938. Three specimens ( LACM-AHF-2580 ), Monterey Bay, Calif., 1 Mar. 1931, G.E. MacGinitie coll. Ten mature specimens ( LACM-AHF-2579 ) , Palos Verdes , California, intertidal, on S. purpuratus, Feb. 1955 . One specimen ( USNM-52640 ) , Stimpson Beach , San Francisco, California, subtidal, 17 Mar. 1957, E. Leise, coll. Two living specimens ( LACM-AHF unumb.), on juvenile sea urchins, 17 mm diam., off Santa Barbara , Calif., 30 Jan. 2002, S. Anderson, coll. On S. franciscanus: Two specimens ( LACM-AHF-2588 ), Point Fermin , Calif., 14 Jan. 1949, J. L. Mohr, coll. One specimen ( LACM-AHF-2578 ), La Jolla, California , 16 m, kelp beds, 1 Feb. 1955, R. Parks coll. On unidentified species of Strongylocentrotus: One specimen ( CAS-10 ), Pigeon Point , San Mateo, Calif., among the spines of sea-urchin, Nov. 1968, P. Carlstroem, coll. Twelve specimens ( LACM-AHF-2551 ) , Portuguese Bend , Calif., 8 Dec. 1938, shore. Twelve specimens ( LACM-AHF-2552 ) , Flat Rock Point , south of Redondo, Calif., 18 Nov. 1941, intertidal. Four specimens ( LACM-AHF-2553 ) , Corona del Mar ( Newport Harbor), 18 Dec. 1941, intertidal. Four specimens ( LACM-AHF-2554 ) , Portuguese Bend , Calif, 13 Mar. 1942, intertidal. Three specimens ( LACM-AHF-2550 ) , Point Arguello , USCG Life Boat Station, 13 Mar. 1942, intertidal. One specimen ( LACM-AHF-2591 ) , Kaiser Point , Monterey, Calif., 3 Feb. 1947, intertidal. One specimen ( LACM-AHF-2581 ) , 1.1 miles from Kelp Point , Port San Bartolome , Baja California Sur, using Derris root in tide pool, surf grass washings, 11 Feb. 1954. Three specimens ( LACM-AHF-5560 ) . One specimen ( LACM-AHF-2577 ) , San Vicente, Calif. , 3 Mar. 1935, Korzinosky, coll. Two specimens ( LACM-AHF-2586 ), Laguna Beach , Calif., 24 Jan. 1948, tide pool, J.L. Mohr coll. Three specimens ( LACM-AHF-2587 ), Cabrillo Beach , Calif., 11 Apr. 1946, J.L. Mohr, coll. Three specimens ( LACM-AHF-2580 ), Cabrillo Beach , Calif., Dec. 1945, on sea-urchin test, M. Parker coll. Three specimens ( LACM-AHF-2590 ), Redondo Beach , Calif., 1 Oct. 1945, J.L. Mohr coll.
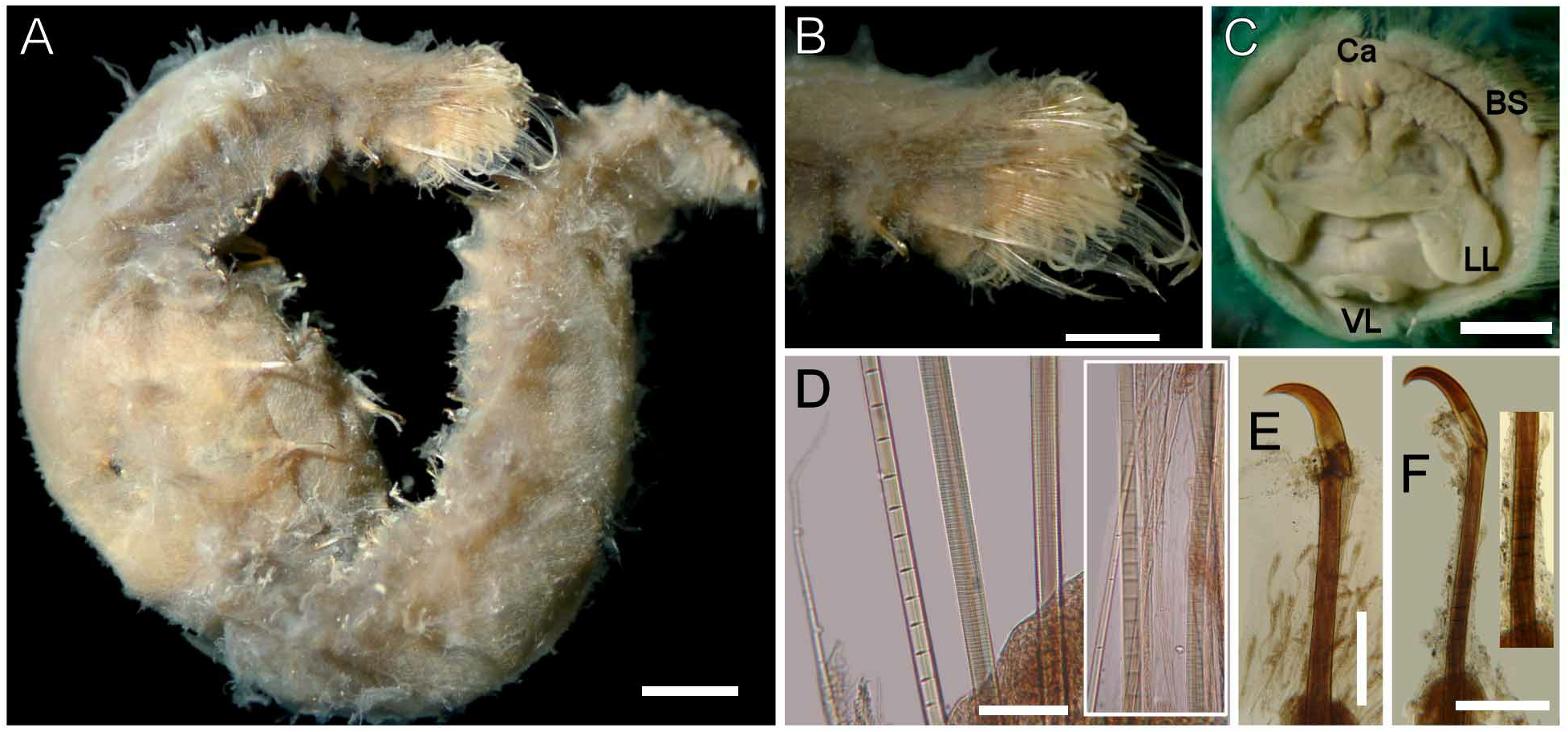
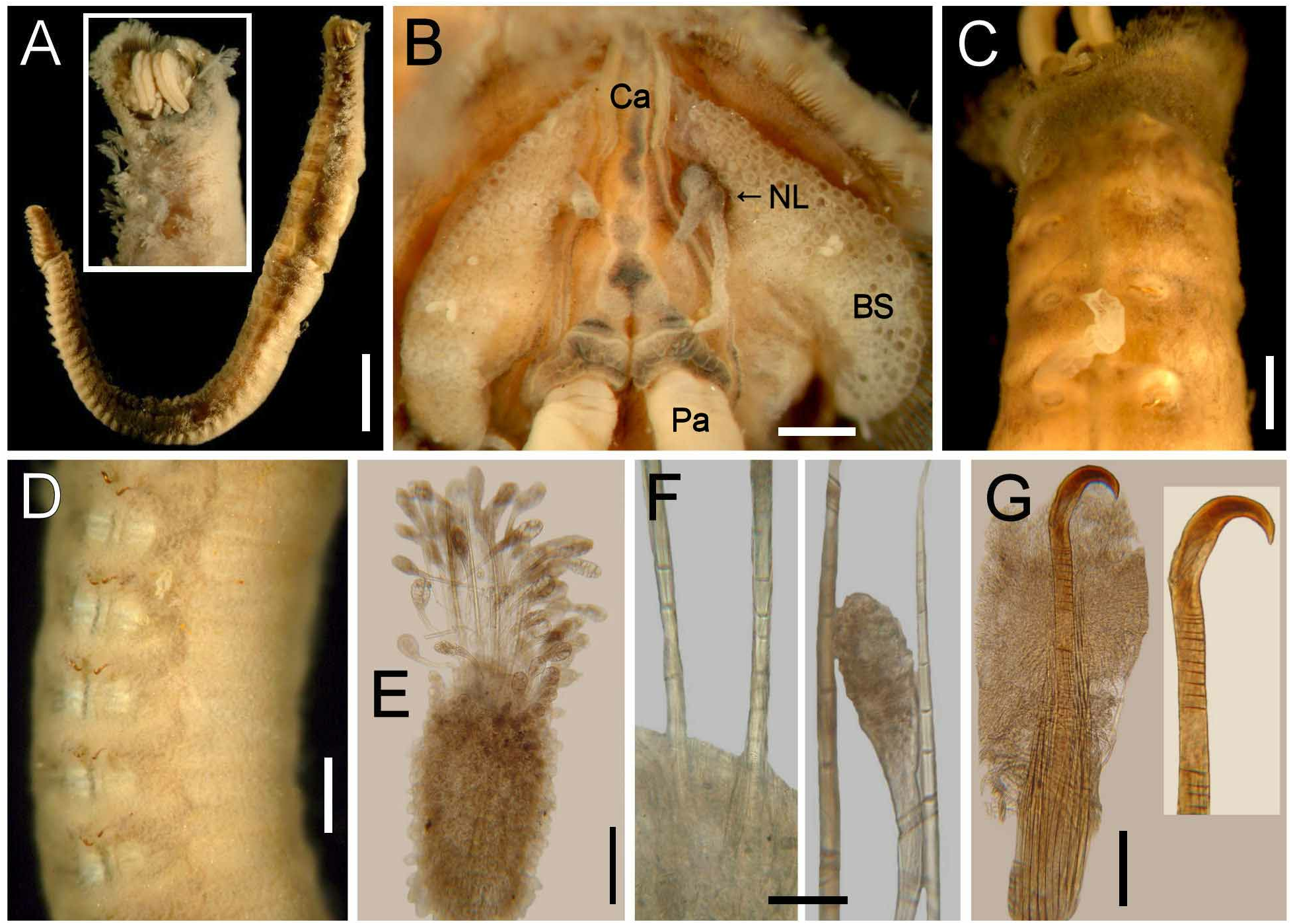
Description. Holotype (CAS-20706) complete, slightly damaged, head distorted, broken anteriorly by visceral eversion ( Fig. 22A View FIGURE 22 ). Body compressed, slightly tapered posteriorly; 47 mm long, 3 mm wide, cephalic cage 1 mm long, 90 chaetigers. Dorsum dark brown, especially over the anterior region (most specimens with a wide middorsal longitudinal dark band not reaching notopodial papillae); first chaetiger with two transverse dark bands, band on following chaetigers faded brown, paler posteriorly, laterally, and ventrally. Tunic thin, not covering the body papillae, free from sediment. Dorsal and lateral papillae fusiform or clavate, with short thin peduncle.
Anterior end observed in paratype (ANSP-2532). No cephalic hood. Prostomium low cone, four slightly pigmented eyes. Caruncle well developed, separating the branchial groups, projected beyond the branchial plate, darker along the midline ( Fig. 22B View FIGURE 22 ). Palps thick, without pigment, palp bases low, rounded.
Branchiae separated into two lateral groups, each with about 100 filaments; branchiae barely exposed from cephalic cage, shorter, thinner than palps, branchial size decreases laterally towards mouth. Nephridial lobes placed laterally on branchial plate, each side with two long filaments, one longer than other, slightly darker than branchiae.
Cephalic cage chaetae 1/47 body length or half body width, only chaetiger 1 involved in cephalic cage, about 50 noto- and 50 neurochaetae per bundle. Anterior dorsal margin of chaetiger 1 papillated. Anterior chaetigers without especially long papillae. Chaetigers 1–3 decreasing in size posteriorly. Chaetal transition from cephalic cage to body chaetae abrupt; neurohooks from chaetiger 2. Gonopodial lobes not seen in holotype; one paratype ( ANSP-2532 ) with everted tube in chaetiger 5 ( Fig. 22C View FIGURE 22 ); another paratype ( CAS-10 ) with everted tubes in chaetigers 5 and 6. Other sexually mature specimens (LACM-AHF-n5557) without exposed tubes .
Parapodia reduced; notopodia dorsolateral, neuropodia ventrolateral up to about chaetiger 7, then displaced ventrally ( Fig. 22D View FIGURE 22 ). Median notopodia separated from each other, over the body corners. Median neuropodia ventral. Notopodia short projected lobes, about as wide as each segment (most eroded but less damaged towards the posterior end in holotype), with many long clavate papillae, covering chaetae, forming a fan-shaped lobe ( Fig. 22E View FIGURE 22 ). Neuropodia less developed, small conical lobe with smaller, filiform clavate papillae, partially covering the hooks. Noto- and neuropodia widely separated.
Median notochaetae arranged in short longitudinal rows, diverging as in a fan, all multiarticulated capillaries, pale, about as long as 1/6 body width, 7–8 per bundle, with long articles ( Fig. 22F View FIGURE 22 ). Neurochaetae multiarticulated capillaries in chaetiger 1; from chaetiger 2 large multiarticulated neurohooks, one per ramus (rarely 2). Handle articulation basally placed, a single long article. Other articles anchylosed, small, continuing to the bending region ( Fig. 22G View FIGURE 22 ). Crest markedly falcate, slightly wider than handle, dark brown along body, pigmentation extending towards the base; width:length ratio 1:4.
Posterior end slightly tapered, pygidium with dorsal anus, with a terminal rounded lobe (less pronounced in holotype), without anal cirri.
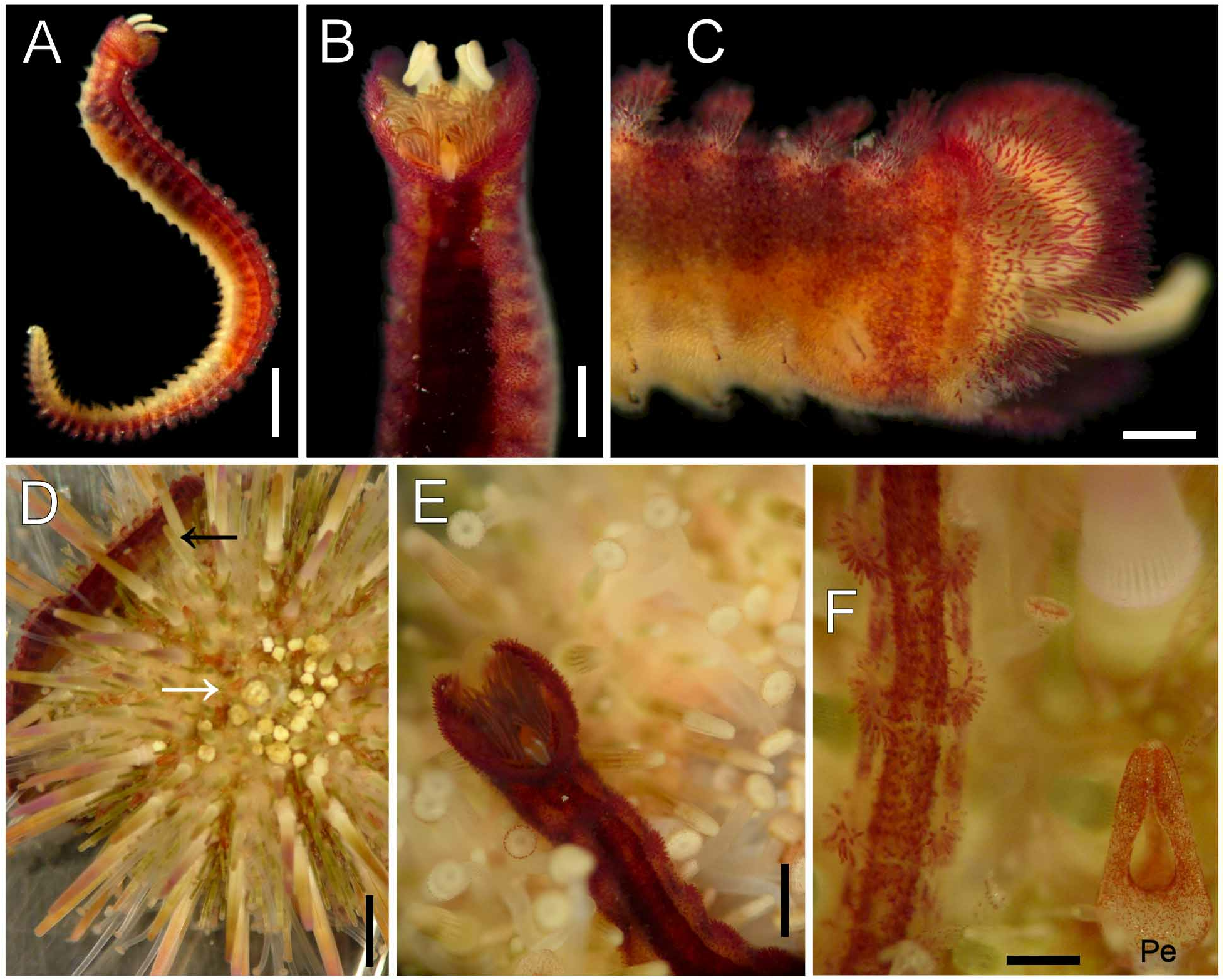
Living specimens. Body reddish dorsally, pale ventrally ( Fig. 23A View FIGURE 23 ). Smaller specimens less colorful. Reddish pigment concentrated in a wide, longitudinal band dorsally and in papillae bulbs ( Fig. 23B, C View FIGURE 23 ), fading lateroventrally and in posterior chaetigers ( Fig. 23F View FIGURE 23 ); neurohooks slightly exposed, dark brown. Palps whitish, branchiae greenish or brownish ( Fig. 23B, E View FIGURE 23 ). The flabelligerid moves easily over the sea urchin trying to avoid light ( Fig. 23D View FIGURE 23 ), but only whips its body when separated from the sea urchin, being incapable of regular walking movements in a Petri dish. The worm’s pigmentation differs strikingly from the juvenile sea urchin spines and body wall appendages ( Fig. 23D, E View FIGURE 23 ), suggesting that it might be associated with larger sea urchins but took shelter in smaller specimens. This is because larger worms resemble more heavily pigmented adult sea urchins, as confirmed with other living specimens. Further, flabelligerid pigmentation also differs from the sea urchin fecal pellets, which are either whitish or dirty yellow ( Fig. 23D View FIGURE 23 ) and might be ingested by the worm, and from the abundant ambulacral feet ( Fig. 23E View FIGURE 23 ). It is noteworthy that flabelligerid pigmentation resembles what is found on pedicellarian epithelium ( Fig. 23F View FIGURE 23 ), and probably over the sea urchin body wall, because it is dark reddish or purple, which is an indirect indication of mimetic pigmentation with, or ingestion of, epithelial layers.
Remarks. Flabesymbios commensalis (Moore, 1909) n. comb. resembles F. roberti n. sp. because both live between sea urchins spines and share several morphological features to hold themselves on the urchin. They differ in pigmentation patterns and in the host sea urchin genus they inhabit; thus, F. commensalis has a basically bicolored body, with a darker back and sides and a pale venter, and lives on Strongylocentrotus , whereas F. roberti n. sp. is homogeneously dark brown or black, including the venter, and lives on Centrostephanus .
The head of F. roberti n. sp. (see below) was studied by Spies (1975, Pl. 2, Fig. 2 View FIGURE 2 , as F. commensalis ) but nephridial lobes were not illustrated. Larger specimens collected on S. franciscanus have genital lobes or slits on chaetiger 5 and some specimens have an elongated tube everted from the lobe or slit. Thus, there may be two different species, each living on a different sea urchin species. Sexually mature specimens also show some differences. One large specimen collected in November (CAS-10) is very large and mature, with almost all internal body space occupied by the gonads. There are other specimens collected in December (LACM-AHF-906), but they are not as large as the previous one and are not sexually mature. Ten sexually mature specimens collected on S. purpuratus in Palos Verdes (LACM-AHF-unnumbered) lack ventral lobes; most have their gonads exposed because the body wall is ruptured. Ovaries are dark and each oocyte is projected from the mass, making the ovary look like a grape cluster, whereas testes are pale, smooth ovoid masses. Another alternative is that the gonopodial lobes develop and decline rapidly, since some sexually mature specimens lack ventral lobes; however, since the larger specimens were found on the larger sea urchin ( S. franciscanus ), and the sea urchins are further ecologically segregated, the flabelligerids might eventually be shown to differ in their reproductive patterns. The type series comes from S. purpuratus , and if further differences are found with those living on S. franciscanus , a new name would be required for them.
In the original description of F. haerens, Chamberlin (1919a:16) relied upon the relative position of neuropodia and the color of the cephalic cage chaetae to separate it from F. commensalis . These features, however, can be modified depending on body contraction and pigmentation. Further, the type specimen (MCZ-2163) has been dried out for a long time; placing it in alcohol with 5% glycerin failed to rehydrate it. The notopodial lobes are rather flat, which could be due to dehydration. Because this species was found in kelp holdfasts, in contrast to living on sea urchins, there might be a difference between those forms living on Strongylocentrotus and those that live elsewhere. However, the intensity of dorsal pigmentation is quite variable and the appearance of the notopodial papillae depends on the proper handling and preservation of the specimens. Therefore, since there are no significant different characters stated in the original description nor in the type specimen, F. haerens is regarded as a subjective junior synonym of F. commensalis . Finding specimens on kelp holdfasts might imply a detachment from the sea urchin in the sampling gear, on deck, or in the lab, especially if the sea urchins were dropped over hard surfaces.
There are no ecological studies about the ratio between flabelligerids and sea urchins, but the abundance of the polychaetes might depend on the abundance of other crustacean symbionts. These crustaceans are an isopod ( Colidotea rostrata ) and a shrimp ( Betaeus macginitieae ); the ratio between the isopods and sea urchins has been well documented ( Stebbins 1988, 1989), but the ratio with shrimps, or the potential interaction among the symbionts, has not been studied at all.
Distribution. Central California, U.S.A. to northwestern Baja California Sur, Mexico, associated with sea urchins ( Strongylocentrotus franciscanus and S. purpuratus ) in low intertidal and subtidal rocky bottoms.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Flabesymbios commensalis (Moore, 1909)
| Salazar-Vallejo, Sergio I. 2012 |
Flabelliderma commensalis :
| Hartman, O. 1969: 286 |
Flabelligera haerens Chamberlin 1919a:16
| Hartman, O. 1938: 14 |
| Chamberlin, R. V. 1919: 16 |
Flabelligera commensalis
| Blake, J. A. 2000: 10 |
| Loi, T. N. 1980: 137 |
| Light, W. J. 1978: 682 |
| Stasek, C. R. 1966: 11 |
| Hartman, O. 1961: 117 |
| Gunther, K. 1912: 16 |
| Moore, J. P. 1909: 288 |