Dryopteris wallichiana (Spreng.) Hyl.
|
publication ID |
https://doi.org/ 10.5252/a2011n1a1 |
|
persistent identifier |
https://treatment.plazi.org/id/886CAA78-FFEC-FFC3-FD69-0F1DFC30FDED |
|
treatment provided by |
Carolina |
|
scientific name |
Dryopteris wallichiana (Spreng.) Hyl. |
| status |
|
Dryopteris wallichiana (Spreng.) Hyl. View in CoL
Botaniska Notiser 3: 352 (1953). — Aspidium wallichianum Spreng. , Systema vegetabilium, 16th ed., 4, 1: 104 (Jan. 1827), non C.Presl ex Kunze (1851: 291). — Aspidium paleaceum D.Don , Prodromus florae nepalensis : 4 (1825), nom. illeg. (McNeill et al. 2006: Art. 53.1), non Lag. ex Sw. (1806). — Aspidium donianum Spreng. , Systema vegetabilium, 16th ed., 4,1: 320 (1827), nom. superfl. (McNeill et al. 2006: Art. 53.1). — Dryopteris doniana (Spreng.) Ching, Sunyatsenia View in CoL 6: 3 (1941), nom. illeg. —
Dryopteris paleacea Hand. -Mazz., Verhandlungen der
Kaiserlich-Königlichen Zoologisch-Botanischen Gesellschaft
in Wien 58: 100 (1908), nom. nov. for Aspidium wal-
lichianum Spreng., nom. superfl. — Type: Nepal , 1820,
N. Wallich [340], (lecto-, B, designated by Fraser-Jenkins
[1989: 353]; isolecto-, BM, G, K, L, P, W, often mixed
collections).
Aspidium paleaceum Lag. ex Sw. , Synopsis filicum : 52 (Mar.-Apr. 1806). — Lastrea paleacea (Lag. ex Sw.) T.Moore , Index filicum 2 (9): 99 (1858). — Dryopteris paleacea (Lag. ex Sw.) C.Chr. , American Fern Journal 1 (5): 94 (7 Aug. 1911), nom. illeg. (McNeill et al. 2006: Art. 53.1), non Hand.-Mazz. (1908: 100). — Type: Peru, M. Lagasca y Segura s.n., Herb. Swartz (holo-, S; iso-, GH, US).
Aspidium parallelogrammum Kunze View in CoL , Linnaea View in CoL 13: 146 (1839). — Dichasium paralellogrammum (Kunze) Fée, Mémoires sur la famille des fougères 5: 302, pl. 23B, fig. 1 (1852). — Nephrodium filix-mas View in CoL (L.) Rich. var. paralellogramma (Kunze) Hook. , Species filicum 4: 116 (1862). — Dryopteris paralellogramma (Kunze) Alston , American Fern Journal 47 (3): 92 (16 Oct. 1957). — Type: Regno Mexico, Hegewisch s.n. & De Karwinski s.n., Herb. Lucae (KIEL, syntypes).
DESCRIPTION
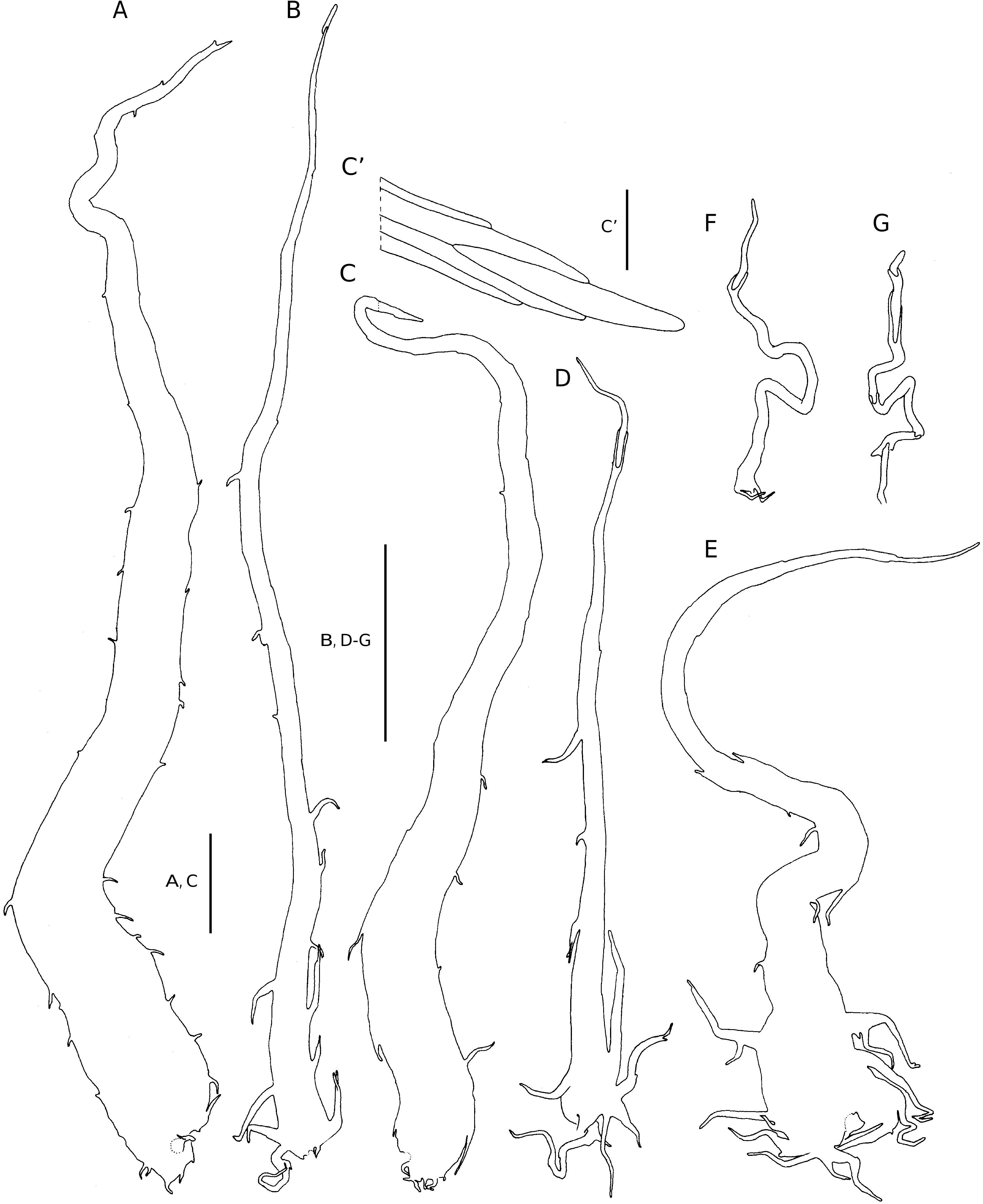
Plants terrestrial. Rhizome short, erect to suberect, up to 150 mm tall and 22 mm in diameter, set with roots, crowded persistent stipe bases and scales, the scales castaneous, chartaceous to thinly crustaceous, broadly attached,subulate, up to 15 × 2mm, cordate, the margins variously set with short and long, apically or basally directed, often branched outgrowths which reduce in number and size towards the scale apex, the scale apex terminates in an elliptic thin-walled cell. Fronds crowded, caespitose, erect to suberect, up to 1.2 m long; stipe proximally castaneous, stramineous higher up, firm, up to 230 mm long, adaxially shallowly sulcate, cicatricate, densely scaled, the smaller scales concolorous, ferrugineous to stramineous, stalked, the larger scales mostly bicolorous, if bicolorous then ferrugineous to stramineous with castaneous streaks, chartaceous, sessile, subulate, up to 17 × 2.4 mm, cordate to cordate-imbricate, the margins proximally variously set with outgrowths similar to those on the rhizome scales, the outgrowths reduce in number and size towards scale apex, the scale apex terminates in a subulate cell or an elliptic thin-walled cell; lamina 1-pinnate-pinnatisect, anadromous, catadromous towards the apex, narrowly elliptic, up to 935 × 270 mm, with up to 47 petiolated pinna pairs, proximally with several pairs of slightly and gradually reducing pinnae; rachis stramineous, adaxially sulcate, the sulcus not open to the sulcus of the costae, cicatricate, moderately to densely scaled, the scales up to 13 × 0.8 mm, the smaller scales mostly concolorous, ferrugineous to stramineous, short-stalked, the larger scales concolorous or bicolorous, if bicolorous then ferrugineous with castaneous streaks, chartaceous, sessile, subulate, cordate to cordate-imbricate, the margins variously set with outgrowths similar to those on rhizome scales, the scale apex terminates in a subulate cell or an elliptic thin-walled cell; pinnae pinnatifid, near opposite to alternate, imbricate or not, the proximal pinnae generally more widely spaced, dark green and glossy adaxially, paler and matt abaxially, up to 140 × 28 mm, oblong-acuminate, petiolate, the petiole up to 2 mm long; costa narrowly winged, adaxially with narrow ridges on either side of the costa, extending between adjacent lateral veins, initially moderately set with ferrugineous, filiform scales, abaxially moderately scaled, the scales ferrugineous to stramineous, chartaceous, sessile to short-stalked, subulate, up to 6 × 1 mm, cordate to cordate-imbricate, the margins irregularly set with outgrowths similar to those on rhizome scales, the scale apex terminates in a subulate cell or an elliptic thin-walled cell; lobes firmly herbaceous, rectangular to parallelogramshaped, up to 16 × 7 mm, the truncate apical margin dentate, the proximal acroscopic lobe often slightly longer than next, proximal basiscopic lobe mostly basiscopically auricled, shallowly lobed (in subsp. madagascariensis ) adaxially glabrous, or sparsely set with filiform scales along the costa and veins, abaxially moderately scaled along the costule, veins and lobe margins, scales along the veins ferrugineous to stramineous,chartaceous,subulate,up to 4 × 0.6 mm, short-stalked, cordate-imbricate, variously set with outgrowths similar to those on rhizome scales, the scale apex terminates in a subulate cell, an elliptic thin-walled cell, or the apical cell undifferentiated, scales along lobe margin, ferrugineous, chartaceous, filiform, up to 5 × 0.1 mm, long-stalked, proximally with short marginal outgrowths, entire distally, the scale apex terminates in a subulate cell, an elliptic thin-walled cell, or the apical cell undifferentiated. Venation evident adaxially and abaxially, lateral veins in lobes forked once or twice to form 2 or 3 veinlets ending near the margin, endings slightly enlarged. Stomata mostly of the polocytic types, (40-)53(-64) µm long. Sori 2-seriate on the lobes, circular, up to 1.5 mm in diameter, discrete at maturity, inframedial on unmodified, predominantly anadromous vein branches; indusium up to 1.5 mm in diameter, brown, firmly chartaceous, reniform or 2-lobed, entire. Sporangium stalk simple, or with a 1- or 2-celled glandular hair, capsule obovate, annulus with (13-)14(-17) indurated annulus cells, epistomium 4(-5)-celled, hypostomium 4(-6)-celled. 32 spores per sporangium, rugate, brown, endospore (40-)48(-54) µm long (Tryon & Lugardon 1990: 423, figs 159.4, 159.5).
REMARKS
Tryon & Tryon (1982: 504) treated Dryopteris wallichiana as a species confined to the palaeotropics, whilst D. paleaceae is considered as a sister species restricted to the neotropics. Others, however, consider them conspecific (Smith & Fraser-Jenkins 1982; Fraser-Jenkins 1986, 1989; Stolze 1981). Dryopteris wallichiana has a world-wide tropical and subtropical distribution with its centre of diversity in SW China and the eastern Himalayas (Fraser- Jenkins 1989). Fraser-Jenkins (2006: 109-110) recognises three subspecies in the D. wallichiana complex, one of them occurring in the study area. It has been shown to be a diploid apomict whilst D. wallichiana subsp. reichsteinii from Zimbabwe is a triploid apomict (Widén et al. 1996: 71). The phloroglucinol derivatives of the species have been studied by Widén et al. (1996).
Dryopteris wallichiana (Spreng.) Hyl.
subsp. madagascariensis (C.Chr.) J.P.Roux ( Figs 1 View FIG ; 2 View FIG )
Synopsis of the Lycopodiophyta and Pteridophyta of Africa, Madagascar and Neighbouring Islands: 124 (Mar. 2009). — Dryopteris paleacea (Lag. ex Sw.) C.Chr. var. madagascariensis C.Chr. , Catalogue des plantes de Madagascar, Pteridophyta: 27 (Feb. 1932), nom. illeg. (McNeill et al. 2006: Art. 32.1[d]). — Dryopteris paleacea (Lag. ex Sw.) C.Chr. var. madagascariensis C.Chr., Dansk Botanisk Arkiv 7: 35, t. 11, figs 5-9 (Oct. 1932). — Dryopteris reichsteinii Fraser-Jenk. , Bulletin of the British Museum, Natural History (Botany): 14 (3): 191 (1986). — Type: Madagascar, Mt. Tsaratanana, 2400 m, IV.1924, H. Perrier de la Bâthie 16454 (holo-, BM000801013!; iso-, P00349500!, P00483234!, P00483235!).
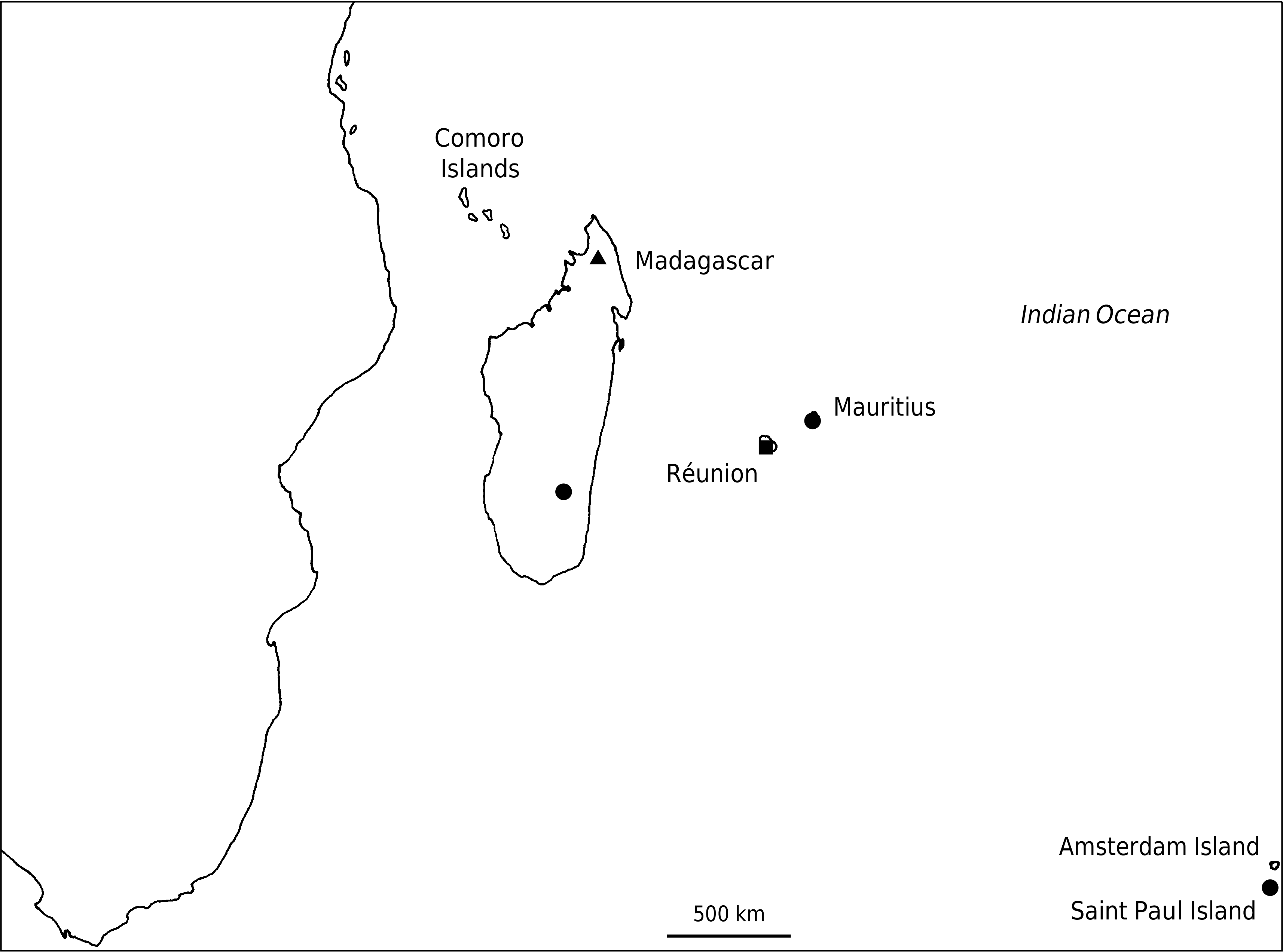
OTHER MATERIAL EXAMINED. — Madagascar. Mont Tsaratanana , 2400 m, IV.1924, Perrier de la Bâthie 16454 (BM000801013; P00349500; P00483234; P00483235) .
Réunion. Habitat in insula Borboniae, Desvaux s.n. (P00349605). — Cilaos, str. du Cap Bouteille, 1740 m, 18.III.2009 (Herb. E. Grangaud).
DIAGNOSTIC FEATURES AND RELATIONSHIPS Dryopteris wallichiana subsp. madagascariensis appears to be generally smaller than the other subspecies as the fronds grow up to 1.1 m long and up to 270 mm wide. The proximal basiscopic lobes are basiscopically auricled and frequently also shallowly lobed. The lamina scales terminate in a subulate cell ( Fig. 2D, F View FIG ), rather than an elliptic thin-walled cell. The larger stoma length, (40-)53(-64) mm, suggests it being a different cytotype than either subsp. wallichiana and subsp. reichsteinii .
DISTRIBUTION AND HABITAT
Dryopteris wallichiana subsp. madagascariensis appears to be rare in the Madagascan region. In Madagascar, it is known from the Tsaratanana Mountains only, where it occurs at 2400 m. At this altitude, the vegetation consists of evergreen forests with an understory consisting of small trees. More recently, the species was rediscovered in La Réunion, occurring at 1740 m in seasonally wet open montane forests ( Fig. 5 View FIG ).
REMARKS
Christensen (1932a: 27) first mentioned the name Dryopteris filix-mas subsp. madagascariensis un- der D. paleaceae , but the former was not validly published (McNeill et al. 2006: Art. 32.1[d]). It was, however, validly published later ( Christensen 1932b: 35). Fraser-Jenkins (1986: 191) raised this variety to species level as D. reichsteinii . He later determined material from the eastern highlands of Zimbabwe to be a triploid apomict and described this as D. wallichiana subsp. reichsteinii Fraser-Jenkins, 1996 (in Widen et al. 1996: 71). I found material from Madagascar to differ from both subsp. wallichiana and subsp. reichsteinii . For this reason, the name Dryopteris wallichiana subsp. madagascariensis was proposed, based on D. paleacea var. madagascariensis C.Chr.
A 1774 Commerson collection said to have come from “Île de France ”, now Mauritius, is housed in the Lamarck Herbarium, Paris (P00307037 & P00307038). Poiret (1804) based Polypodium umbilicatum on this collection. It was later transferred to Aspidium Sw. ( Desvaux 1811: 320, 321), and still later to Nephrodium Rich. as a variety of N. marginale Mich. ( Desvaux 1827: 260) . The name then appears to have been forgotten as it was not cited for nearly a century until Morton (1973: 265) commented on the type, tentatively ascribing it to D. filix-mas (L.) Schott, but suggested that it may be D. paleacea var. madagascariensis . The origin of the collection is clearly erroneous as the Commerson material is that of D. filix-mas , a species not occurring on Mauritius.
Dryopteris Adans. sect. Lophodium (Newman) C.Chr. ex H.Itô
Nova flora japonica 4: 65 (1939). — Lophodium Newman in New Phytologist 4: 371, app. XVI (1851). — Dryopteris Adans. View in CoL group Lophodium (Newman) C.Chr. , Index filicum: XXI (1905) . — Lectotype: Lophodium spinulosum (O.F.Muell.) Newman ( Polypodium spinulosum O.F.Muell. ), designated by Christensen (1905: XXI), now Dryopteris carthusiana (Vill.) H.P.Fuchs. View in CoL
Aspidium Sw. group Spinulosa H.Christ, Farnkräuter der Erde: 261 (1897), nom. nud. (McNeill et al. 2006: Art. 32.1[d]). — Type: none indicated. Two species – A. spinulosum Sw. and A. aemula (Soland.) Sw. are listed. Aspidium spinulosum View in CoL may be considered as the type.
REMARKS
A group of about 10 species chiefly occurring throughout the northern temperate regions of the world, Macaronesia, and northern Africa, with few species extending to the southern hemisphere.
Diagnostic features of the section are the short, erect or decumbent rhizome that is closely and irregularly branched and which is set with roots and closely spaced trophopods. The lamina is up to 3-pinnate and the lobes are strongly dentate. The fertile vein branches are frequently shortened and end in the sori, or they extend for a distance beyond the sorus. Indumentum is composed of glands, moniliform hairs, and scales, the scale apex and marginal outgrowths generally end in a long moniliform series of cells. Fraser-Jenkins (1986: 195) noted that the minutely spinulose perispores may be unique for the section.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Dryopteris wallichiana (Spreng.) Hyl.
| Roux, Jacobus P. 2011 |
Aspidium
| H. Christ 1897: 261 |