Rhinolophus ferrumequinum, Schreber, 1774
|
publication ID |
https://doi.org/10.5281/zenodo.3748525 |
|
DOI |
https://doi.org/10.5281/zenodo.3808956 |
|
persistent identifier |
https://treatment.plazi.org/id/885887A2-FFDF-8A38-F899-FBD8F436D1A0 |
|
treatment provided by |
Plazi |
|
scientific name |
Rhinolophus ferrumequinum |
| status |
|
31 View On . Greater Horseshoe Bat
Rhinolophus ferrumequinum View in CoL
French: Grand Rhinolophe / German: Grosse Hufeisennase I Spanish: Herradura grande
Other common names: Larger Horseshoe Bat
Taxonomy. Vespertilioferrum-equinum Schreber, 1774 View in CoL ,
France .
Rhinolophusferrumequinum is in the ferrumequinum species group with the extinct species R maghrebensis and R mellali . The ferrumequinum group is included in the Afro-Palearctic clade of Rhinolophus close to the maclaudi , fumigatus , and xinanzhongguoensis groups. Rhinolophusferrumequinum is sister to R clivosus , although some Egyptian specimens attributed to R clivosus cluster within R ferrumequinum . Rhinolophus nippon was previously included as a subspecies of R ferrumequinum , but it is genetically sister to the clade including R ferrumequinum and R clivosus . Exact distributional limit between R nippon and R ferrumequinum is currendy uncertain due to lack of genetic data from specimens in central and southern Asia, and thus, distributional difference here is tentatively marked at Kashmir. Subspecies creticumis, now considered a synonym of the nominate form whereas subspecies Irani is now a synonym of proximus, although genetic tests have not been performed on populations from central and southern Asia. Two subspecies recognized.
Subspecies and Distribution.
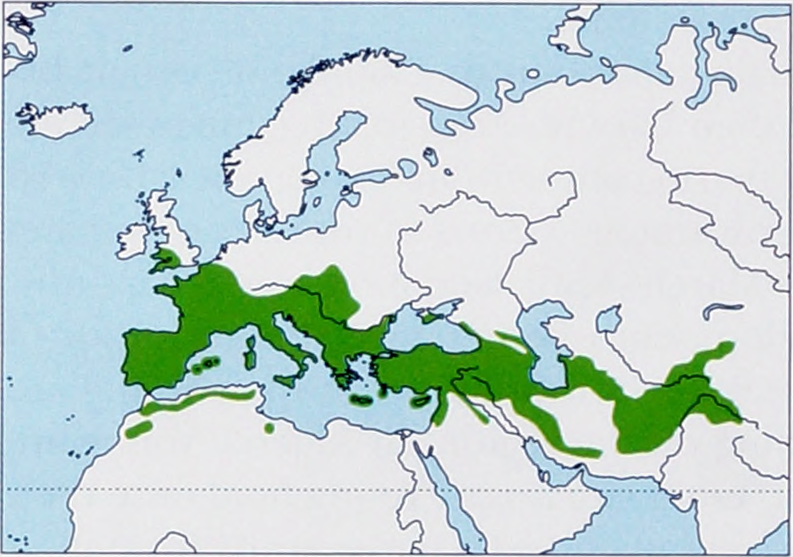
R f. ferrumequinum Schreber, 1774 - S Europe from Iberian Peninsula and France E to Romania, Bulgaria, and Greece including SW Great Britain, S Germany, S Poland, and Crimea, also on most Mediterranean Is (Balearics, Corsica, Sardinia, Sicily, Malta, Crete, Cyprus, etc.), Turkey ( Anatolia), NW Africa (N Morocco, N Algeria, N Tunisia, and NW Libya), and Levant region.
R f. proximus K. Andersen, 1905 - Transcaucasia, Mesopotamia, S Turkmenistan, N, W & S Iran, Afghanistan, Tajikistan, Kyrgyzstan, Pakistan, and N India (Kashmir). View Figure
Descriptive notes. Head-body 54-71 mm, tail 31-44 mm, ear 19-25 mm, hindfoot 10- 14 mm, forearm 51-61 mm; weight 13-44 g. The Greater Horseshoe Bat is the largest rhinolophid in Europe. Nominate ferrumequinum is generally larger than proximus. Dorsal pelage is generally grayish brown to pale brown (hairs are beige, with grayish brown or brown tips) and variably, usually lightly, tinged with red; venter is grayish white to yellowish white. Juveniles are ashy gray. Specimens of proximus are generally pale fawn. There is no orange morph. Males lack axillary tufts. Ears are medium to short ( c.41% of forearm length). Noseleafhas hastate or subtriangular lancet, becoming slightly concave near bluntly pointed tip; connecting process is rounded and much higher than tip of sella; sella is naked, relatively small, and curved forward, making front surface strongly concave, and sides are only slightly concave, and tip is pointed; and horseshoe is narrow at 6-5-9- 9 mm, does not cover muzzle, and has lateral leaflets (although sometimes inconspicuous) and deep median emargination. Lower lip has one or three grooves, and lateral grooves can be inconspicuous if present. Wings and uropatagium are pale brown or grayish brown. Baculum has dorso-ventrally flattened basal cone, with somewhat deep, ventral incision; rim of cone is thickened and forms strong protuberance on both sides ofventral incision; shaft tapers and is almost cylindrical; and tip is a dorso-ventrally strongly flattened lancet Skull is robust, with sturdy zygomatic arches (zygomatic width is greater than mastoid width); nasal swellings are ofmedium low height; frontal depression is shallow; supraorbital crests are weak; and sagittal crest is well developed anteriorly but absent posteriorly. P2 is tiny and fully displaced labially or absent, allowing C1 and P4 to touch, and P3 is also tiny and fully displaced labially or absent, so that P2 and P are in full contact Dental formula is variable: 11/2, C 1/1, P 2/2, M 3/3 (x2) = 30; 11/2, C 1/1, P 1/2, M 3/3 (x2) = 28; 11/2, C 1/1, P 2/3, M 3/3 ( x 2) = 32; or 11/2, C 1/1’, P 1/3, M 3/3 (x2) = 30. Chromosomal complement has 2n = 58 and FNa = 60-62.
Habitat Mainly forested habitats, especially in areas with abundant roosting sites, from sea level to elevations of c. 3500 m. Unlike the related Geoffroy’s Horseshoe Bat ( clivosus ), the Greater Horseshoe Bat is less associated with dry habitats, although it is found in some drier regions through West Asia and North Africa. In Europe, it is found in deciduous temperate woodlands throughout the northern part of its distribution and more Mediterranean and sub-Mediterranean woodlands and shrublands in southern Europe, North Africa, and the Levant Greater Horseshoe Bats are typically associated with forested habitats where they forage near vegetation, but they can be found in open areas, especially in West Asia. Throughout western and central Asia, they are largely associated with highland habitats and only found in temperate forested montane regions of Transcaucasia, Iran, and central Asia from Afghanistan to Kashmir.
Food and Feeding. Greater Horseshoe Bats forage by slow hawking, fly-catching from perches, and ground-gleaning. When captured, insects are eaten on the wing or taken to a perch. They will sometimes land on the ground to capture dung beetles and other insects in dung piles. Most of their diet comes from species of Lepidoptera, Coleoptern , Hymenoptera , and Diptera (at least throughout Europe), but other arthropods also make up smaller proportions of their diets, including Neuroptera, Trichoptera, and Araneae . A large sample of 1580 feces found that the diet of the Greater Horseshoe Bat in the UK mostly contained Lepidoptera (moths) and changed throughout the year. In spring, they ate cockchafers {Melolontha melolonthd), beetles ( Geotrupe), caddisflies (Trichoptera), tipulid flies, ichneumonid wasps, and moths. In autumn, dung beetles (Aphodius) and dung flies (various Diptera ) replaced various species of beetles in diets. Coleoptera, Lepidoptera , and Diptera dominated diets in Azerbaijan, and Coleoptera and Lepidoptera were most important in Turkey, Syria, and Jordan. Moths dominated three stomach samples from Iran, and some brachyceran Diptera, Trichoptera , and scarabaeid Coleoptera also were eaten. Greater Horseshoe Bats are versatile feeders and alter their diets based on prey availability throughout the year and among regions. Where they forage also can change; in the UK, they forage mainly in woodlands in spring and over pastures in late summer.
Breeding. Greater Horseshoe Bats are seasonally monoestrous. Copulation occurs before hibernation; males mate with females in their harems. After mating, males secrete a plug into females’ vaginas to either prevent sperm competition or keep sperm stored in the female until the following spring. Sperm storage occurs in oviducts of females until ovulation and fertilization takes place in March—April. Gestation lasts 2—3 months depending on whether or not embryo development is lengthened due to torpor of the pregnant female; gestation is generally c.9 weeks with limited torpor. Young are bom in late spring or early summer depending on the region. In Algeria, young are bom inJune and volant by the end ofAugust. Litter size is one. Young hold onto their mothers until a little before weaning. Young will forage and hang separate from their mothers before weaning. Greater Horseshoe Bats mature very slowly, similar to many hibernating bats. Females reach sexual maturity at c.3 years of age, but they might not produce their first offspring until 3—5 years of age. Males generally mature at 2-4 years of age. Greater Horseshoe Bats are long-lived; maximum longevities recorded in the wild are 30 years and six months for a male and 27 years and eight months for a female.
Activity patterns. Greater Horseshoe Bats are nocturnal and forage throughout the night They generally leave day roosts 15—30 minutes after sunset and begin to forage. They typically linger around day roosts for the early part of the night and fly further from the day roost as the night progresses. Most nightly activity occurs within 5—20 km ofroosts, and adults typically move further thanjuveniles. Greater Horseshoe Bats generally stay out all night or return after 2—3 hours and leave day roosts again for c.30 - 50 minutes before sunrise. They occasionally rest in night roosts. Activity decreases in cold temperatures, markedly below 10°C—the typical thermal threshold for insect activity. During the day, Greater Horseshoe Bats can enter a mild torpor when temperatures are below 22°C. They enter deep torpor (hibernation) from mid-autumn until spring (October-April in Europe), during which they find parts of caves that are warmer than 7 — 10 ° C. During this time, they continue to irregularly awake and leave roosts to forage when weather permits. They do not hibernate in southern parts of the distribution but have been found to hibernate in Iran. Day roosts of Greater Horseshoe Bats are generally in caves and other underground or rocky structures; they commonly inhabit abandoned or unused parts of buildings, particularly roofs. Greater Horseshoe Bats tend to favor houses in northern parts of their distribution but underground structures in the south. They also seem to favor caves as hibernacula in winter. Call shape is FM/ CF /FM, with terminal FM sweep usually having the greatest bandwidth. Aspects of calls vary among seasons and through the lifetime of an individual; juveniles emit lower frequencies than adults. Resting frequencies of mothers and young seem to be similar, indicating that the call is learned from the mother or inherited. F component is 77—83 kHz in southern Europe, 83—84 kHz in the UK, 81-7 kHz in Western Europe, and 84-6 kHz in Morocco. Mean call duration is 53-8 milliseconds in Greece, 21 milliseconds and 31-9 milliseconds in the UK, and 30-3 milliseconds in Morocco.
Movements, Home range and Social organization. Greater Horseshoe Bats are highly gregarious, roosting singly, in small groups, or in very large colonies up to 1000 individuals, although they forage alone. Non-matemity colonies have been recorded with up to 500 individuals of both sexes; summer maternity colonies of up to 1000 females have been reported, although 100—300 individuals are more common. Maternity colonies are createdjust before females give birth. When first formed, maternity colonies can still include some persistent males and non-breeding individuals that usually leave after young are bom. Non-breeding individuals disperse like males or can remain in maternity colonies throughout the breeding season. Males form scattered small groups in separate day roosts. All male and all juvenile colonies have been recorded. Males and females start roosting together after young are weaned around late summer (at least in Europe), which leads to copulation in day roosts until the end of October before the beginning of hibernation (in northern populations). From late summer to about mid-autumn, males become territorial in roosting colonies, establishing small harems in the roost. Harems generally include the male and up to eight females, segregating into a single cluster in the roost Populations of Greater Horseshoe Bats are primarily sedentary and hibernate instead of migrating. Nevertheless, they often fly 20—35 km between summer roosts and hibernacula. They typically move to more secluded localities ( e.g. cool, deep parts of caves) where they can enter deep torpor. Individuals wrap themselves completely in their wing membranes during hibernation. Hibernacula generally include fewer individuals than summer roosts but can sometimes include clusters ofup to 100 individuals. Females seem to give birth in the same roosting area each year. Non-matemity summer roosts are often shared with other species of Rhinolophus , Miniopterus , Myotis , Asellia , and Plecotus . Maternity colonies are occasionally shared with other bat species.
Status and Conservation. Classified as Least Concern on The IUCNed List. The Greater Horseshoe Bat has a wide distribution and is rather abundant throughout much of its distribution. Nevertheless, there are areas that have experienced well-documented declines, including Malta, Belgium, and the Netherlands where it is likely extinct Much of the documented decline has occurred throughout Europe, notably in north-western Europe. Populations in the UK have experienced massive declines, and the overall population is now stable at a low level of c.5000 individuals. Austrian populations have declined by 70% in the last ten years and are down to only c.30 breeding individuals. Population trends throughout the rest of Europe and North Africa are uncertain, but roosts seem to be disappearing throughout the Iberian Peninsula. Populations are considered stable in Croatia. There apparently has been a slow increase in populations in Romania since 1989 due to the reduced use of pesticides. The Greater Horseshoe Bat is rare in Switzerland but considered stable. It is considered relatively rare throughout much of its Asiatic distribution, although it is considerably common throughout Transcaucasia. Primary threats are fragmentation and habitat destruction from deforestation and agricultural expansion. Use of pesticides also negatively affects Greater Horseshoe Bats by targeting some important food sources such as melolonthid beetles, noctuid moth larvae, and crane flies. General roost disturbance is also an evident threat, especially for colonies in buildings where human intolerance can make it difficult for cohabitation. The Greater Horseshoe Bat is widely protected throughout Europe, where many underground roosts have been protected and building roosts have had management agreements to maintain the bat-human relationship. Legislation protects the Greater Horseshoe Bat in some but not all countries in its distribution.
Bibliography. ACR (2018), Aldridge (1986), Arslan & Zima (2014), Aulagnier &Thévenot (1986), Bates & Harrison (1997), Benda & Gaisler (2015), Benda & Vallo (2012), Benda, Abi-Said et al. (2016), Benda, Andreas et al. (2006), Benda, Faizolâhi et al. (2012), Benda, Georgiakakis et al. (2008), Benda, Hanâk & Cervenÿ (2011), Benda, Ivanova et al. (2003), Benda, Lucan et al. (2010), Benda, Spitzenberger et al. (2014), Botnariuc &Tatole (2005), Csorba et al. (2003), Disca et al. (2014), landers, Jones et al. (2009), landers, Wei Li et al. (2011), Gaisler (2001, 2013b), Gunnell et al. (2011), Hanâk et al. (2001), Jones (1990), Jones & Ransome (1993), Jones & Rayner (1989), Jones & Siemers (2011), Koh Hung-Sun et al. (2014), Long & Schnitzler (1975), Ma Jie, Kobayasi et al. (2006), Park et al. (2000), Piraccini (2016a), Ransome & McOwat (1994), Rossiter, Benda et al. (2007), Rossiter, Jones et al. (2000a, 2000b, 2001), Schnitzler (1973), Schnitzler & Grinnell (1977), Stoffberg et al. (2010), Suga et al. (1976), Vogler & Neuweiler (1983), Walters et al. (2012).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Rhinolophus ferrumequinum
| Burgin, Connor 2019 |
Vespertilioferrum-equinum
| Schreber 1774 |