Benedenia fieldsi, Deveney, Marty R. & Whittington, Ian D., 2010
|
publication ID |
https://doi.org/10.5281/zenodo.275591 |
|
DOI |
https://doi.org/10.5281/zenodo.6203148 |
|
persistent identifier |
https://treatment.plazi.org/id/82668798-FF8D-FFFF-FF33-5C6AFAC0FCED |
|
treatment provided by |
Plazi |
|
scientific name |
Benedenia fieldsi |
| status |
sp. nov. |
Benedenia fieldsi View in CoL n. sp.
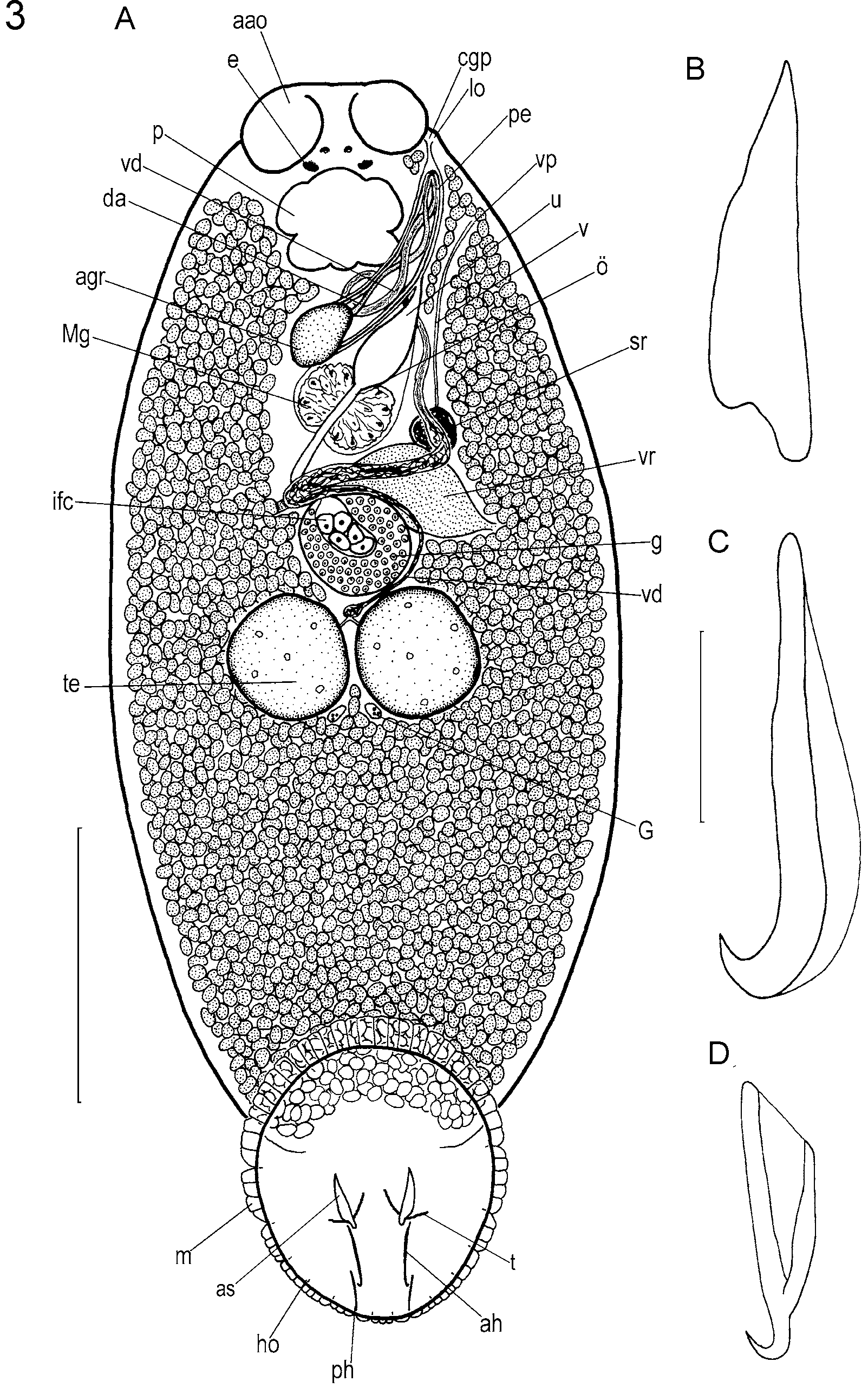
( Figs. 3 View FIGURE 3 , 4)
Synonym: undescribed benedeniine of Whittington (1996; see his figs. 1 and 2).
Type host and locality: Cephalopholis boenak (Bloch) ( Perciformes : Serranidae ), Heron Island (23°27ˏS, 151°55ˏE), Queensland, Australia.
Other hosts and localities: Cephalopholis cyanostigma (Valenciennes in Cuvier & Valenciennes); Cephalopholis miniata (Forsskål) ( Perciformes : Serranidae ), Heron Island (23°27ˏS, 151°55ˏE), Lizard Island (14°40ˏS, 145°27ˏE), Queensland, Australia.
Other hosts in aquaria: Epinephelus cyanopodus (Richardson) ; Epinephelus fasciatus (Forsskål) ; Epinephelus ongus (Bloch) ; Epinephelus polyphekadion (Bleeker) ; Epinephelus quoyanus (Valenciennes) ; Epinephelus tauvina (Forsskål) ; Plectropomus leopardus (Lacépède) ; Plectropomus laevis (Lacépède) ; Pseudanthias huchtii (Bleeker) ; Variola louti (Forsskål) ( Perciformes : Serranidae ).
Site on host: Fins, particularly the regions with soft rays. In heavy infections in aquaria, site specificity breaks down and we observed individual parasites on the skin, head and buccal surfaces.
Holotype: QM: G 218896 ex dorsal fin C. boenak, Heron Island.
Paratypes: QM: G 218897–906 (10 slides, 10 specimens) ex fins C. boenak, Heron Island.
Voucher specimens: SAMA 29684–98 ex fins C. cyanostigma, Lizard Island (14°40ˏS, 145°27ˏE), Queensland, Australia. SAMA 29699–703 ex fins C. boenak, Heron Island (23°27ˏS, 151°55ˏE), Queensland, Australia
Etymology: The specific name honours Mr Charley J. “Chuck” Fields, who assisted the senior author with field work and translated papers from Spanish into English.
Adult description and observations: Based on studies of many living specimens and 10 wholemounts of preserved, sexually mature specimens. Measurements from all wholemounted types unless indicated otherwise. Total length including haptor 557–1087 (848); maximum breadth 230–681 (425) at level of testes ( Fig. 3 View FIGURE 3 A). Haptor elliptical, longer than wide: 200–407 (266) long, 149–341 (220) wide. Accessory sclerite 25–75 (49) long, broad proximally, straight, tapering with pointed distal tips ( Fig. 3 View FIGURE 3 A, B). Anterior hamulus 49–90 (66) long, narrow proximally, broader distally with wing-like dorsal extension, strongly curved distal hook ( Fig. 3 View FIGURE 3 A, C). Posterior hamulus 42–58 (47) long, root and shaft broad, tapering to a pointed, strongly curved distal hook ( Fig. 3 View FIGURE 3 A, D). Fourteen sickle-shaped hooklets, each 4–7 (5) long (n=20). Marginal valve scalloped with consistent pattern of lobes between hooklets on each side of haptor: 3 small lobes between hooklets of pair II on posterior border of haptor; 1 lobe between hooklets II and position of posterior hamuli; 3 lobes between posterior hamuli and hooklets of pair III; 3–4 lobes between hooklets III and IV; 2–3 lobes between hooklets IV and V; 3–4 lobes between hooklets V and VI; 4–5 lobes between hooklets VI and VII; 8– 10 lobes between hooklets VII and VIII; 10–12 lobes between hooklets of pair VIII on anterior border of haptor ( Fig. 3 View FIGURE 3 A).
Anterior attachment organs ellipsoidal, relatively small, approximately 75–133 (93) in diameter. Three adhesive zones observed in living material (e.g. see Whittington & Kearn 1993). Pharynx 41–114 (76) long, 51–157 (98) wide. Two pairs of eyespots, pigment shielded, dorsal, between pharynx and anterior margin of body. Unclear whether gut caeca confluent posteriorly. Body and haptor usually heavily pigmented (Fig. 4). Pigment ranges from light pink or orange to deep red and occasionally almost black.
Glands of Goto in posterior angle between testes ( Fig. 3 View FIGURE 3 A). Vas deferens swells to form seminal vesicle between testes and germarium ( Fig. 3 View FIGURE 3 A). Penis muscular. Vas deferens joins penis canal dorsally. Vas deferens and duct of male accessory gland reservoir twisted together along length of penis in regular pattern, joining near distal tip of penis ( Fig. 3 View FIGURE 3 A). Penis protrusible via common genital duct and common genital pore. Small lobe near common genital pore ( Fig. 3 View FIGURE 3 ).
FIGURE. 4. Light photomicrographs of Benedenia fieldsi n. sp.. A. Whole living animal, ventral view, showing pigment contained in the body, haptor and anterior attachment organs. B. Anterior region, dorsal view, showing somatic pigment and pigmented eyespots. Abbreviations: pi, pigment. Other abbreviations as in previous Figures. Scale bars: A, 250 Μm; B, 125 Μm.
Germarium globular, compact with large internal fertilisation chamber ( Fig. 3 View FIGURE 3 A). Uterus short ( Fig. 3 View FIGURE 3 A). Vaginal duct a plain tube, opening submarginally and dorsally, posterior to common genital pore at level of middle of pharynx. Vaginal pore unremarkable. Vaginal duct expands proximally to form seminal receptacle. Seminal receptacle communicates with vitelline reservoir by short, narrow duct. Vitelline follicles extend in body proper from level of pharynx posteriorly to a position at level of anterior third of haptor ( Fig. 3 View FIGURE 3 A). Eggs tetrahedral with sides 58–118 (103) long (n=20); elongate filamentous appendage from one non-opercular pole.
Larval observations: We examined oncomiracidia of B. fieldsi from specimens removed from aquaria containing heavily infected host fish. When viewed by phase-contrast light microscopy, the larva of this species is indistinguishable from those of Benedenia lutjani Whittington & Kearn, 1993 and B. rohdei (see Whittington et al. 1994).
Comments and differential diagnosis: Benedenia fieldsi is similar to Benedenia epinepheli ( Yamaguti, 1937) Meserve, 1938 and Yamaguti’s 4 Hawaiian Benedenia species: Benedenia bodiani Yamaguti, 1968 , Benedenia hawaiiensis Yamaguti, 1968 , Benedenia lolo Yamaguti, 1968 and Benedenia scari Yamaguti, 1968 placing it among the most difficult Benedenia species to discriminate and identify. Yamaguti’s 4 Hawaiian species and B. epinepheli , however, are larger in most respects than B. fieldsi and the anterior hamuli of B. fieldsi differ from these other species in being broad distally and with a straight taper so they narrow proximally. The accessory sclerites and hamuli of B. fieldsi resemble those of B. epinepheli , but are located more anteriorly on the haptor than in B. epinepheli . Benedenia fieldsi can further be differentiated from B. epinepheli as redescribed by Ogawa et al. (1995) because it lacks the swelling in the distal two-thirds of the penis, the lobe associated with the vaginal pore and the muscular, cup-shaped distal region of the vagina. The lobe we observed near the common genital pore in B. fieldsi is smaller and more symmetrical ( Fig. 3 View FIGURE 3 A) than the corresponding lobe in B. epinepheli . Ogawa’s specimens of B. epinepheli differ from B. fieldsi by possessing a marginal valve comprising single large lobes between the hooklets, whereas the marginal valve of B. fieldsi comprises many small lobes. The anterior attachment organs of B. fieldsi are proportionally smaller than those of B. epinepheli . The anterior hamuli distinguish B. fieldsi from B. scari : in B. scari the anterior hamuli have a broader proximal end. The lack of prominent wavy musculature in the haptor of B. fieldsi further differentiates it from B. scari and from B. bodiani . Benedenia scari can also be differentiated from B. fieldsi by the posterior position of the large sclerites on the haptor. The distal region of the penis of B. fieldsi is not tapered, as is the penis of B. hawaiiensis (see Whittington et al. 2001; see also below). Furthermore the large haptoral sclerites of B. fieldsi are proportionally smaller than those of B. hawaiiensis and B. lolo . Benedenia lolo also has a haptor that is larger in comparison to body size. Pigmentation was not referred to by Yamaguti (1968), likely because he did not observe live or recently dead parasites. Benedenia fieldsi is the smallest species in the genus with a mean total length including the haptor of 848 µm.
| SAMA |
South Australia Museum |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |