Vinca minor, L.
|
publication ID |
https://doi.org/10.1016/j.phytochem.2021.113017 |
|
DOI |
https://doi.org/10.5281/zenodo.8239997 |
|
persistent identifier |
https://treatment.plazi.org/id/7F122842-D13A-FFEA-FFFD-F8DDFAFEF82E |
|
treatment provided by |
Felipe |
|
scientific name |
Vinca minor |
| status |
|
2.1. Phytochemical investigation of Vinca minor View in CoL View at ENA
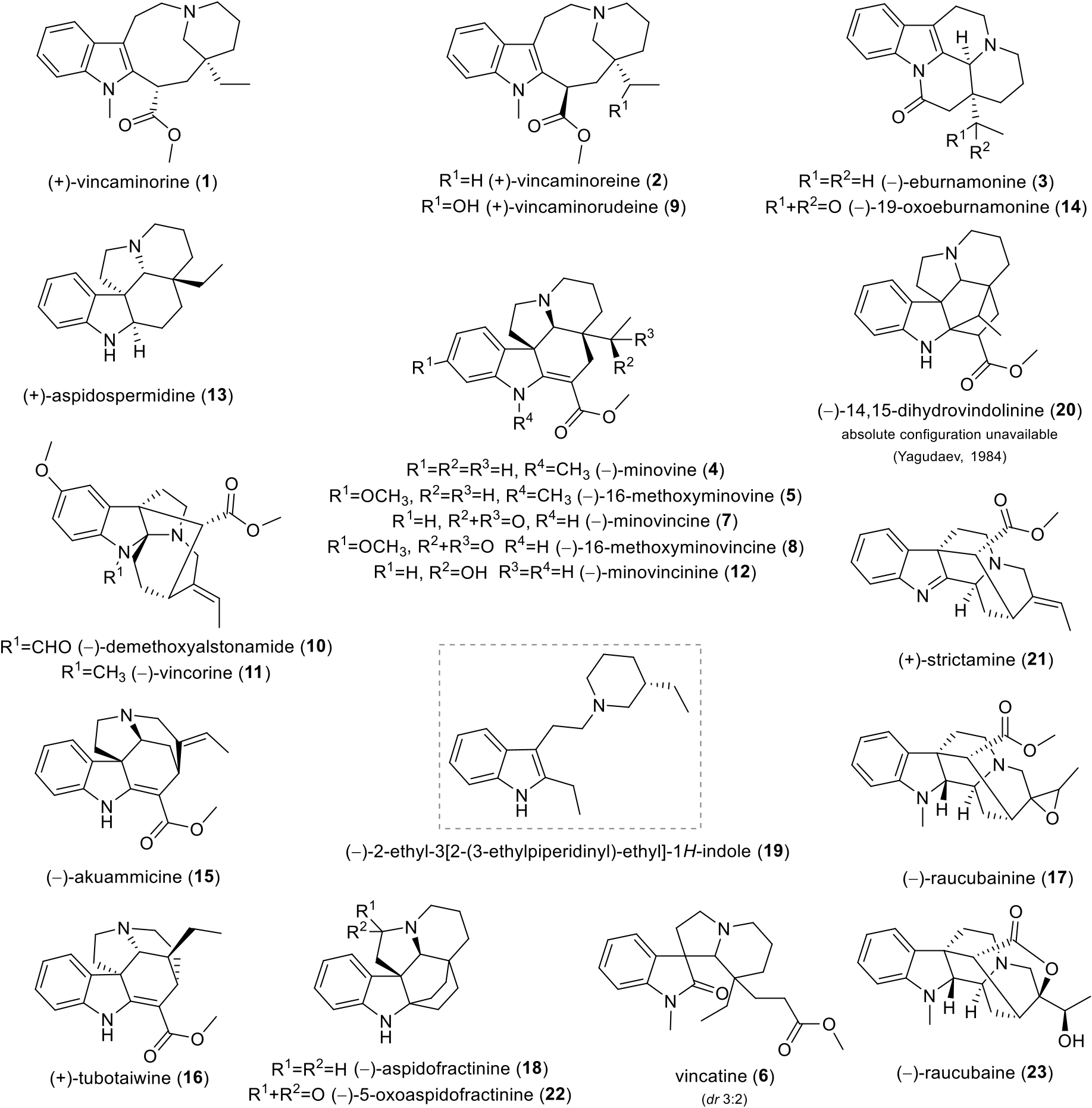
GC-MS analysis of the alkaloid extract of V. minor led to the initial identification of monoterpene indole alkaloids. Moreover, this extract showed interesting human cholinesterases inhibition activities (IC 50, hAChE = 191.58 ± 38.03 μg/mL; IC 50, h BuChE = 13.60 ± 0.82 μg/mL). The promising bioactivities of the indole alkaloids, together with the absence of a detailed up-to-date phytochemical report on V. minor , encouraged us to examine this species. Extensive chromatographic purification led to the isolation of twenty-two known and one undescribed indole alkaloids ( Fig. 1 View Fig ). The known alkaloids were identified by comparison of their MS, ESI-HRMS, 1D and 2D NMR data with the literature as vincaminorine ( 1) (Farahanikia et al., 2011; Tan et al., 2016), eburnamonine ( 3) (Kovacik and Kompiˇs, 1969; Li et al., 2019), minovine ( 4) (Farahanikia et al., 2011; Tan et al., 2016), vincatine ( 6, diastereoisomers 3:2) (Ali et al., 1982; Danieli et al., 1984; D¨oepke et al., 1969), minovincine ( 7) (Farahanikia et al., 2011; Kalaus et al., 1997; Laforteza et al., 2013; Varga et al., 2020), demethoxyalstonamide ( 10) (Ur-Rahman et al., 1991a), vincorine ( 11) (Horning and MacMillan, 2013; Mokrý et al., 1962), minovincinine ( 12) (D¨oepke and Meisel, 1970; Plat et al., 1962; Williams et al., 2019; Zeng et al., 2020), aspidospermidine ( 13) (Kim et al., 2017; Ma et al., 2015; Xu et al., 2019), 19-oxoeburnamonine ( 14) (Kitajima et al., 2014), akuammicine ( 15) (Hong and Vanderwal, 2017), tubotaiwine ( 16) (Martin et al., 2011), aspidofractinine ( 18) (Varga et al., 2020), 2-ethyl-3[2-(3-ethylpiperidinyl)-ethyl]-1 H -indole ( 19) (Ur-Rahman et al., 1991b), 14, 15-dihydrovindolinine ( 20) (Yagudaev, 1984), strictamine ( 21) (Chen et al., 2018), and 5-oxoaspidofractinine ( 22) (Wenkert and Liu, 1994). The NMR data for previously described alkaloids vincaminoreine ( 2) (Farahanikia et al., 2011), 16-methoxyminovine ( 5) (Kuehne et al., 1979), 16-methoxyminovincine ( 8) (Plat et al., 1962), raucubainine ( 17) (Sierra et al., 1982), and raucubaine ( 23) (Sierra et al., 1982) have been revised, corrected and missing data added. The newly isolated alkaloid was named vincaminorudeine ( 9). Alkaloids 5, 10, 13, 14, 16, 17–20, 22, and 23 are herein reported for the first time in this species.
Alkaloid 10 was previously isolated from Alstonia macrophylla Wall. ex G. Don (Ur-Rahman et al., 1991a) . Alkaloid 13 was isolated from Tabernaemontana bufalina Lour (Shi et al., 2019) . and Rhazya stricta Decne. (Abdel-Mogib et al., 1998) , as well as alkaloid 18 and its structural types, which can be also found in Kopsia spp. (Kitajima et al., 2014; Wong et al., 2021). Kopsia spp. is also a source of alkaloid 14 (Kitajima et al., 2014). Alkaloid 16 was obtained, for example, from T. bufalina (Shi et al., 2019) , as well as from Haplophyton crooksii L. D. Benson (Mroue et al., 1996) . Rauvolfia salicifolia Griseb. is a source of alkaloids 17 and 23 (Sierra et al., 1982). Alkaloid 23 can also be found in Alstonia costata (G. Forst.) R. Br. (Jacquier et al., 1982) . Alkaloid 20 was previously isolated from other Vinca spp. (Abdurakhimova et al., 1967). All of the mentioned plants belong to the Apocynacae family. So far, alkaloids 5 and 22 have not been found in a natural source, but were prepared synthetically (Kuehne et al., 1979; Wenkert and Liu, 1994). The most active alkaloid ( 19) is discussed in detail later.
The undescribed monoterpene alkaloid ( 9) exhibited a molecular ion peak [M+ H] + at m/z 371.2339, matching the formula C 22 H 30 N 2 O 3 + (calc. 371.2329). The compound was isolated as light brown crystals, but, unfortunately, not in sufficient quantity for X-ray diffraction analysis. Moreover, none of the circular dichroism data for compounds with the required similarity were available.
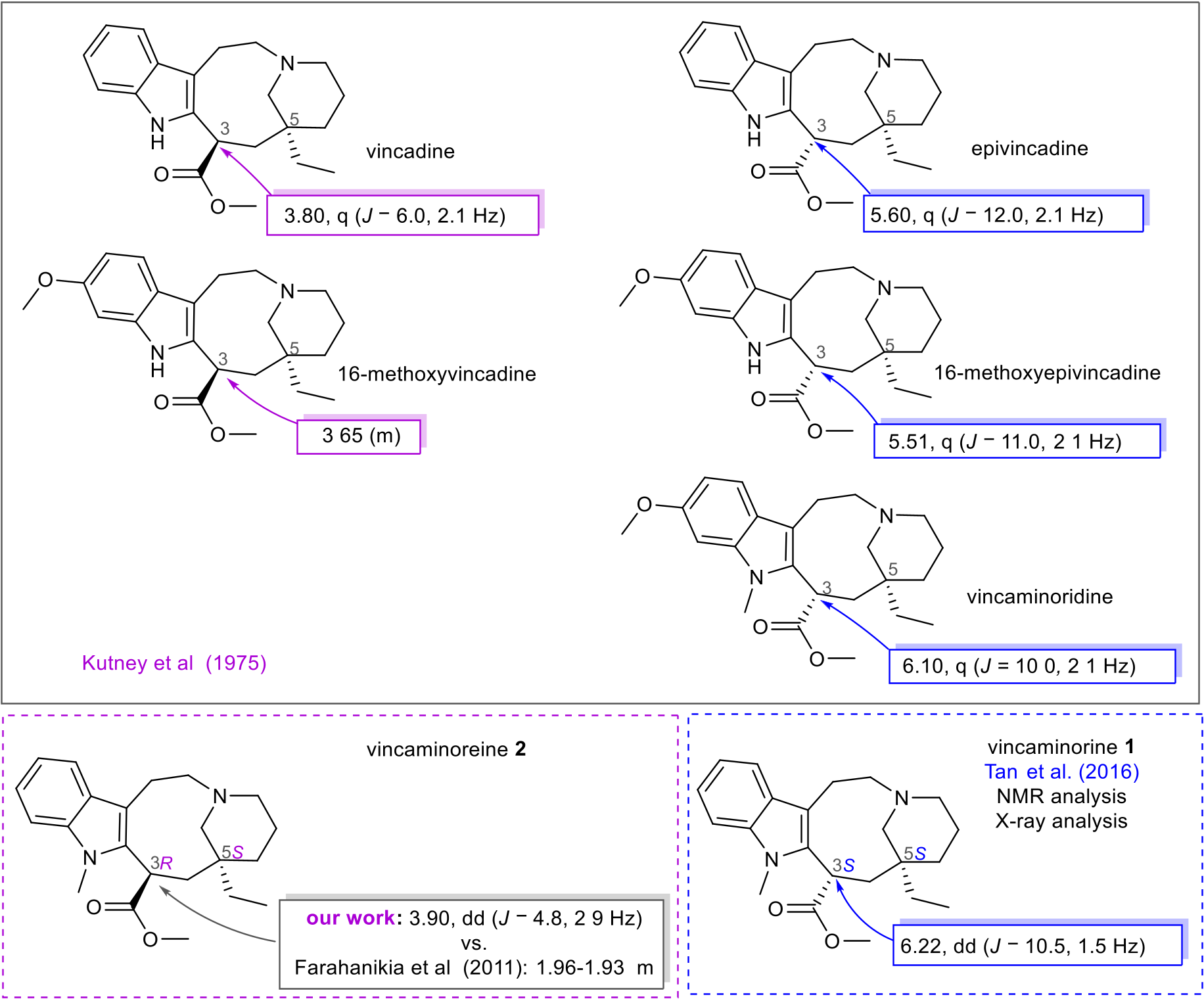
However, the chemical constitution of 9 was elucidated by NMR analysis. The 1 H NMR spectrum revealed four signals of aromatic protons with a characteristic splitting pattern for a 1,2-disubstituted benzene ring ( δH 7.53, dd, J = 7.8 Hz, J = 1.1 Hz, H-14; 7.27, dd, J = 7.8 Hz, J = 1.1 Hz, H-17; 7.19, td, J = 7.8 Hz, J = 1.1 Hz, H-16; 7.09, td, J = 7.8 Hz, J = 1.1 Hz, H-15), and one methine α- proton ( δH 4.39, dd, J = 4.3 Hz, J = 2.7 Hz, H-3), the chemical shift of which was crucial for determining the relative configuration of this stereocenter (see Table 1 View Table 1 , Fig. 2 View Fig , respectively). A deshielded singlet ( δH 3.72, s, 3-COOCH 3) was also observed, corresponding to a methyl ester group overlapped with a multiplet of a one proton diastereotopic methylene group ( δH 3.75–3.70, m, H-19), as well as characteristic signals of the 1-hydroxyethyl group ( δH 3.47, q, J = 6.5 Hz, H-20; 1.29, d, overlap, J = 6.5 Hz, H-21). The signal of this methyl group overlapped with a multiplet of two protons ( δH 1.36–1.21, m, overlap, H-7, H-6). Resonances of another eight protons were further recognized. In the 13 C NMR spectrum, 21 carbon signals were observed: specific chemical shifts for a methyl ester group ( δC 177.0 and 52.7, 3-COOCH 3), four quaternary sp 2 -carbons ( δC 139.1, C-2; 136.7, C-18; 127.0, C-13; 110.3, C-12), four protonated sp 2 -carbons ( δC 120.7, C-16; 118.5, C-15; 118.1, C-14; 108.6, C-17), a deshielded O - methine group ( δC 74.2, C-20), three N -methylene groups ( δC 56.6, C-19; 55.2, C-8; 53.6, C-10), one quaternary sp 3 -carbon ( δC 42.9, C-5), one α- carbon ( δC 38.7, C-3), four methylene groups ( δC 31.7, C-6; 31.1, C-4; 22.3, C-11; 22.3, C-7), one N -methyl ( δC 30.3, 1- CH 3), and one more shielded methyl ( δC 17.6, C-21). Every individual signal of the proton and carbon NMR spectra was unambiguously assigned to specific groups employing HSQC experiment. Coupled spin systems were identified by correlations in the COSY and H2BC spectra, and then the N -methylindole moiety and hexahydroazonine cycle were established according to correlations found in the HMBC spectrum. This 9-membered ring condensed with indole is characteristic of quebrachamine-type alkaloids. Furthermore, the propylene connection of the tertiary nitrogen and quaternary carbon C-5 was determined by two-dimensional (2D) NMR experiments. The substituent of C-5 was established by HMBC cross-peaks of H-21/C-5 and H-20/C-19. All related key correlations are depicted in Table 1 View Table 1 . Due to several reasons, the relative configuration was determined for all three chiral centers in 9. Unfortunately, the configuration of the hydroxy methylene C-20 must be left unspecified because of a free rotation of the 1-hydroxy ethylene and the impossibility of using NOESY experiment. Moreover, it was not possible to either derivatize or analyze the compound by X-ray, as mentioned above. At least the stereochemistry of the other two carbons was determined. Because of the many conformations of the 1-azabicyclo [6.3.1]dodec-4-ene moiety, NOESY experiment was not used for the determination of its relative configuration. By comparison of the data for an analogue ( 1), a structure confirmed by X-ray crystallographic analysis, with that of its diastereomer ( 2), the relative configuration at C-3 and C-5 was determined for 9 (Tan et al., 2016).
When comparing the chemical shifts of 9 with known analogues without a 20-hydroxy substitution, vincaminorine ( 1) and its 3-epimer vincaminoreine ( 2), to confirm further the configuration of 9, an inconsistency was found in the published data. Farahanikia et al. (2011) presented NMR data for 1 and 2. Both these alkaloids were also isolated in our work, allowing us to compare the chemical shifts of these molecules. The interpretation of our 1D NMR spectra of 1 matched the published data of 1 prepared in the enantioselective synthesis (Tan et al., 2016). X-ray analysis confirmed the configuration of 1 in Tan’ s work (Tan et al., 2016). When the 3-methyl ester and 5-ethyl substituents of 1 are cis orientated (3 S*,5 S*), the chemical shift of the α- proton is more deshielded. The cause of this has been described in the past and is supposed to be the conformation in which this atom is centered onto N 4 (Gilbert, 1968). Otherwise, when these substituents are in the trans position (3 R*,5 S*), the α- proton is more shielded, and it is found in the range of 3.90 to 3.65 ppm. Fig. 2 View Fig shows this assumption in summary for 1 and 2 supported by data of other known related 3-methyl esters. Accordingly, it was possible to elucidate the relative configuration at C-3 and C-5 of compounds 1, 2, and 9.
The NMR data of 2 presented by Farahanikia et al. were different from our experimental findings (Farahanikia et al., 2011), which are summarized in Table 2 View Table 2 . For instance, the chemical shift of α proton (H-3) was described as a multiplet at 1.96–1.93 ppm, but our experimental data, supported by 2D NMR experiments, confirmed the resonance of H-3 at 3.90 ppm, split into a doublet of doublets ( J = 4.8 Hz and 2.9 Hz). In Table 2 View Table 2 , our experimental 1 H and 13 C NMR chemical shifts for 2 are compared with the previously published data. For further proof of this revision of the interpretation of the 1 H and 13 C spectra, a comparison of the chemical shifts of other 3-methyl ester quebrachamine derivatives was made (see Fig. 2 View Fig ).
For four isolated alkaloids, namely 5, 8, 17, and 23, relevant NMR data have not yet been published. Therefore, all compounds were extensively analysed by NMR spectroscopy (1D, and 2D NMR experiments), HRMS, polarimetry, and circular dichroism.
Compounds 5 and 8 both belong to the aspidospermine type of indole alkaloids. Compound 5 was named as (±)- N -methylervinceine (its synthesis was reported in 1979) (Kuehne et al., 1979), and ()-2,3-anhydro-4-deacetoxy-6,7-dihydrovindoline, an intermediate in vindoline synthesis published in 1978 (Kutney et al., 1978). These articles provide MS, IR, UV, [ a] D, and melting point, but only poor interpretations of the 1 H NMR spectrum. Kutney et al. (1978) described the optical rotation for the synthetic intermediate as [α] D = 377.2 (c 0.17, CHCl 3). However, our experimental value was [α] D = 21.2 (c 0.17, CHCl 3). Based on these facts, data of the isolated minovine ( 4), which is a well-described alkaloid (Ishikawa et al., 2006; Tan et al., 2016; Yuan et al., 2005), were used in comparison with its 16-methoxy analog ( 5). Experimental data were correlated with those of 5 (see Table S1 View Table 1 ); accordingly, the name 16-methoxyminovine fits much more properly for structure 5 in the described context .
A similar issue occurred for 8, with two names previously used for this compound. 16-Methoxyminovincine modified from 16-methoxyminovincinine by Oppenauer oxidation of the secondary hydroxyl, was previously described by melting point only, without analysis, but with determined stereochemistry (Doepke ¨and Meisel, 1968). The second name used for 8 is minoriceine from the work of Zachystalova´et al. (1963), which was described as an isolated indole alkaloid from Vinca minor L., with published analytical data for melting point, UV spectrum, and [ a] D. We believe that the compound presented under these names in the ’60s equals 8, the 16-methoxy derivative of the well-known Vinca alkaloid minovincine ( 7), also isolated in our work. According to the comparison of the 1D and 2D NMR data, and the similar optical rotation value of 8 with its analog 7 (see Table S2 View Table 2 ), the name 16-methoxyminovincine has been set for 8.
After 2D NMR confirmation of the unusual picraline type alkaloids raucubainine ( 17) and raucubaine ( 23), only interpretations of 1 H NMR spectra were found in the literature as references. The identified chemical constitution of 17 with an epoxide moiety was found in the structure of raucubainine ( Rauvolfia salicifolia Griseb. , (Sierra et al., 1982)) and quaternoxine ( Alstonia quaternata Van Heurck & Müll. Arg. , (Mamatas-Kalamaras et al., 1975)), previously described diastereomers. The assignment of individual CH 3 / CH 2 / CH /C groups in the original article (Sierra et al., 1982) has been revised (see Table S3 View Table 3 ). Based on our analysis of the 2D NMR spectra, the interchange of methylene groups at positions 6 and 14 was recognized in comparison with the previous assignment. The red-colored bonds in Fig. 3 View Fig show COSY correlations of 3.83 (H-5)/1.69 ppm (H-6) and 3.83 (H-5)/2.72–2.68 ppm (H-6), determining the ethylene connection from the indoline moiety to the second tertiary nitrogen. In addition, the spin-spin system H-2/H-3/H-14/H-15/H-16 was also confirmed in the COSY experiment. Another proof is HMBC correlations of H-6 to the quaternary sp 2 carbon C-8, as well as correlations over three bonds of methylene H-14 to C-2, C-16, and C-20 (see Fig. 3 View Fig ). The results of the NOESY experiment suggest two possible orientations of the epoxide to the rest of the molecule, which was impossible to distinguish without X-ray analysis. Therefore, the configuration at C-19 and C-20 remains undetermined, as in the case with Sierra et al. (1982).
Two diastereomers, raucubaine (Sierra et al., 1982) and quaternoline (Mamatas-Kalamaras et al., 1975), are described for the constitution of 23 as well. Quaternoline was excluded from the comparison due to its poorly described analytical data, as was the case with quaternoxine and alkaloid 17. Nevertheless, the 1 H NMR spectrum presented by Sierra et al. (1982) corresponds to our experimental data perfectly, along with specific optical rotation and circular dichroism data. The configuration of raucubaine was previously determined by X-ray (Pauptit and Trotter, 1981), CD analysis, and polarimetry (Sierra et al., 1982) in one collaboration. When comparing the experimental 1 H NMR spectral details with those published for raucubaine (Sierra et al., 1982), almost identical chemical shifts were observed (see Table S4). The missing 13 C NMR spectrum interpretation of 23, with established stereochemistry of 2 R, 3 S,7 R,15 R,16 R,19 R,20 S, is presented in the Supplementary Data, together with the data of other isolated known alkaloids for which the NMR spectroscopic details have not been fully reported.
2.2. Biological activity of isolated indole alkaloids
All the isolated alkaloids were tested for their inhibition of h AChE and h BuChE. Alkaloids obtained in sufficient amounts were also evaluated for their activity against POP. To determine h AChE and h BuChE inhibition properties, galanthamine and eserine were used as reference compounds. Regarding the POP inhibition assay, berberine served as a reference compound. Results of the biological studies are summarized in Table 3 View Table 3 . In the h AChE assay, all isolated alkaloids were inactive (IC 50 > 100 μM, Table 3 View Table 3 ). On the other hand, some of the alkaloids showed a promising capacity to inhibit h BuChE. Vincaminoreine ( 2) and vincorine ( 11) demonstrated inhibition potential in the single-digit micromolar range with IC 50 values of = 8.71 ± 0.49 μM, and 9.75 ± 0.45 μM, respectively. The best h BuChE inhibition activity was demonstrated by ()-2-ethyl-3[2-(3-ethylpiperidinyl)-ethyl]-1 H -indole (also known as demethoxycarbonyltetrahydrosecodine (Aclinou et al., 1994), 19) with an IC 50 value of 650 ± 16 nM. This structurally simple indole alkaloid has been previously isolated from other Apocynaceae plants (e.g., Haplophyton crooksii L. D. Benson , and Tabernaemontana pachysiphon Stapf ) and tested for its potential to inhibit AChE from electric eel (Crooks et al., 1968; Mroue et al., 1996). The obtained inhibition potency within the previous study (IC 50 = 203 μM) is in agreement with our results (IC 50 = > 100 μM; Table 3 View Table 3 ), pointing to very weak AChE inhibition potency. Compound (+)- 19 was isolated from the leaves of Rhazya stricta Decne. (Ur-Rahman et al., 1991b) . The cholinesterase inhibition activity of the (+)-isomer has not been studied. Since these alkaloids are isolated from natural sources only in a limited amount, the possibility of stereoselective synthesis of the (+)-isomer was successfully explored by Palmisano et al. (1995). Both isomers can also be synthetically prepared with a shorter route by alkylation of phenylglycinol-derived bicyclic lactams (Amat et al., 2005). The preparation of semisynthetic analogues derived from 19 could be interesting for the further development of selective BuChE inhibitors.
For POP inhibition potency, alkaloid 19 was the most active compound in our study. Compound 19 revealed two-digit inhibition ability, with an IC 50 value of 58 ± 0.45 μM ( Table 3 View Table 3 ), being nearly three times more active than berberine, an isoquinoline alkaloid recognized as a POP inhibitor (Tarrago et al., 2007).
Since GSK-3β is involved in the formation of Aβ and NFTs in AD, it can be considered a promising target for developing antidementia drugs. Thus, alkaloid 19, as the most active compound from the cholinesterase and POP inhibition assays, was also screened for its GSK-3β inhibition potency at a concentration of 100 μM. However, it showed only marginal inhibition potential (31.43 ± 3.05% inhibition of GSK-3β).
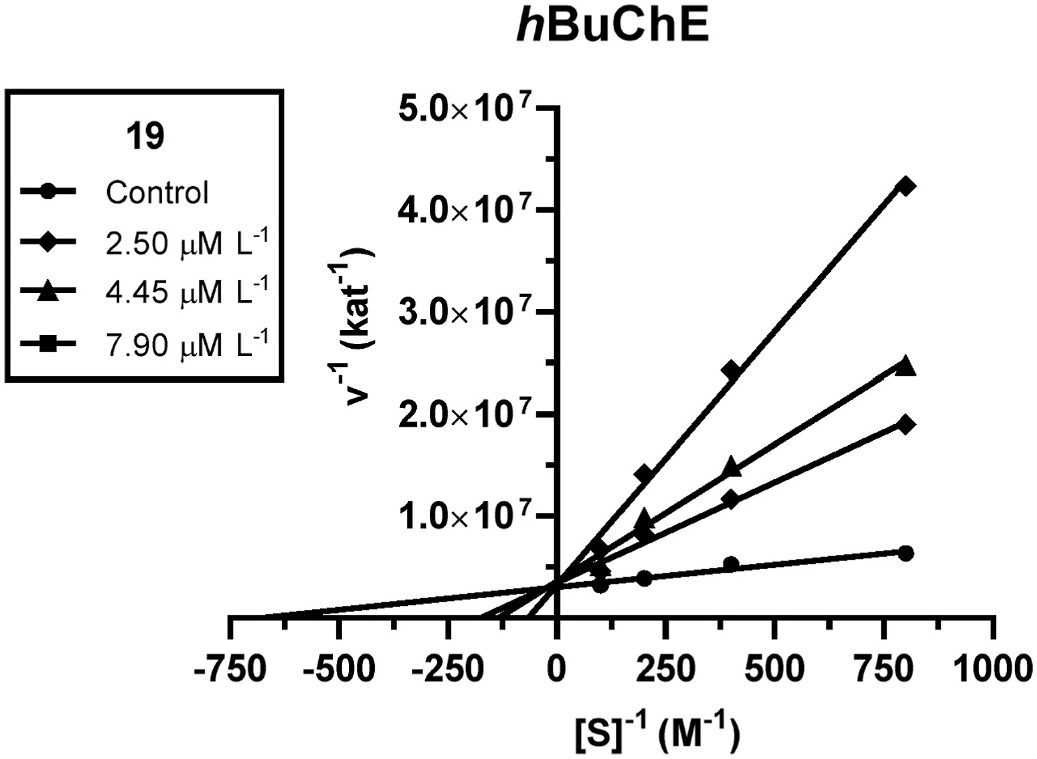
An enzyme kinetic study was carried out to explore the inhibition mechanism of active compound 19 with h BuChE to describe the involved interaction. Inhibition kinetics was determined from velocity curves measured at different concentrations of 19. The type of enzyme inhibition and appropriate affinity parameter ( K i) was calculated by nonlinear regression analysis. Results for each type of model of inhibition (competitive, non-competitive, uncompetitive, and mixed) were compared by the sum-of-squares F-test. Statistical analysis showed a competitive type of inhibition (p ˂ 0.05), which is in accordance with the Lineweaver–Burk (double reciprocal) plot, used for visualization of the obtained data ( Fig. 4 View Fig ).
The intersection of lines is located on the y-axis, which means reversible competitive binding mode to the active site of the enzyme. With increasing concentration of inhibitor, the apparent V max remained unchanged and K m increased. A K i value of 54.9 ± 8.8 nM was determined for 16 on h BuChE.
The crucial requirement for a compound to be of clinical relevance for AD is its CNS availability; thus, the compound must penetrate through the blood-brain barrier. One of the fastest methods to predict that is the calculation of logBB, the logarithmic ratio between the concentration of a compound in the brain and blood. Compounds with logBB > 0.3 have a very high probability of easy penetration by passive diffusion, whereas compounds with logBB < 1 are unlikely to pass; values between 0.3 and 1 still mean the theoretical ability for penetration (Kunwittaya et al., 2013). According to this method, all h BuChE active alkaloids (IC 50, h BuChE < 10 μM) comply with this requirement ( Table 3 View Table 3 ). Another commonly employed method to predict the availability of the compound to the CNS is in vitro parallel artificial membrane permeability assessment (PAMPA). Based on the obtained results for in vitro permeability of 19 ( Pe = 15.7 ± 1.3 × 10 6 cm s 1), it can be concluded that this indole alkaloid can cross the BBB by passive diffusion ( Table 3 View Table 3 ).
2.3. Docking study of ()-2-ethyl-3[2-(3-ethylpiperidinyl)-ethyl]-1H-
indole (19)
A molecular dynamic simulation was performed to determine the structural aspects crucial for the high BuChE inhibition ability of 19. The template structure of h BuChE was taken from Protein Data Bank (PDB ID: 4BDS) (Nachon et al., 2013). The result was compared with a crystal structure of tacrine, the first FDA-approved drug for AD therapy acting as a dual AChE/BuChE inhibitor (Soukup et al., 2013).
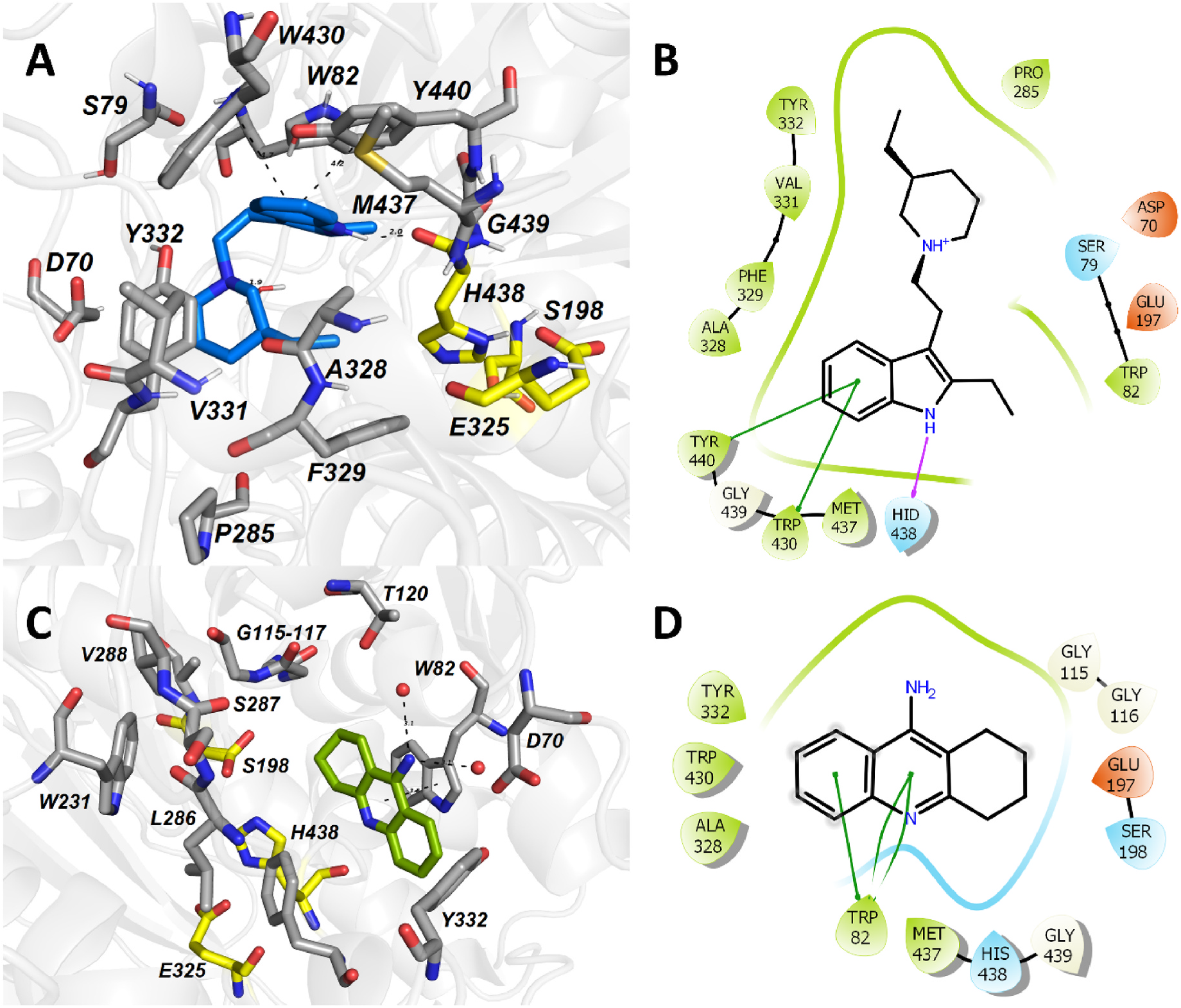
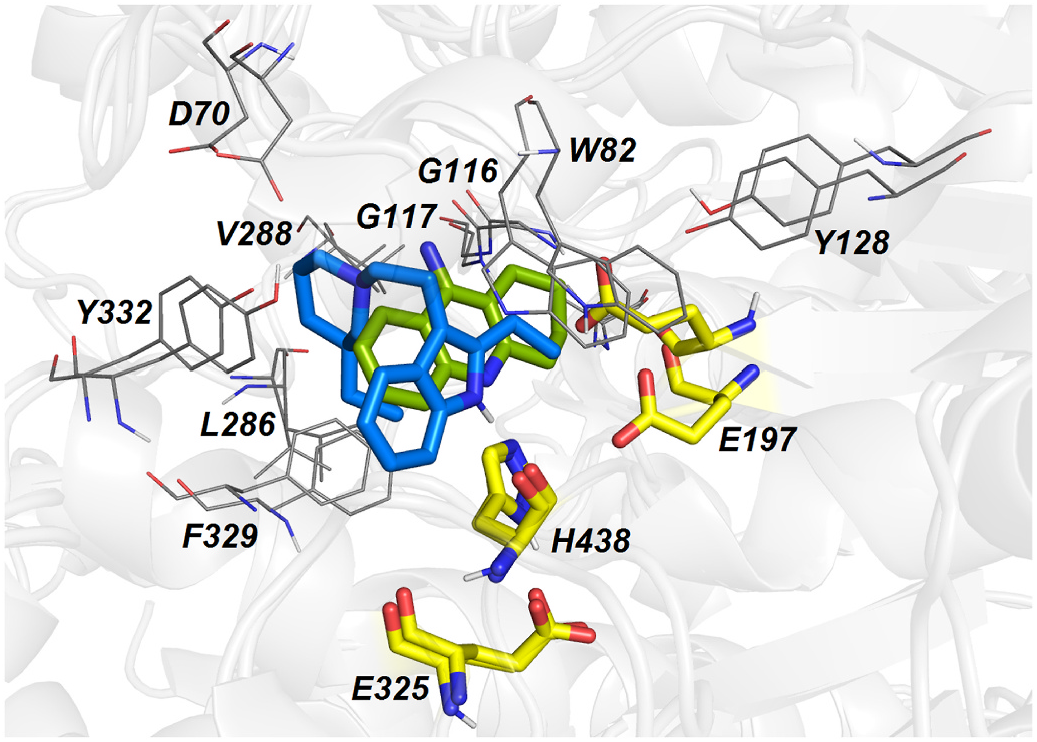
Molecular dynamic simulation of 19 within the h BuChE active site revealed several crucial interactions responsible for high ligand affinity ( Fig. 5A and B View Fig ). The 2-ethyl-1 H -indole moiety of 19 is engaged in parallel π- π and displaced π- π stackings with Y440 (4.2 Å) and W430 (4.7 Å), respectively. Moreover, the 2-ethyl-1 H -indole moiety lies in the vicinity of W82 (4.3 Å). The hydrogen atom of the indole moiety contacts the amide backbone of H438 from the catalytic triad via hydrogen bond interaction (1.9 Å). Other catalytic triad residues (S198, E325) stand aside from 19. The protonated nitrogen atom of the 3-ethylpiperidine moiety faces towards Y332 (4.5 Å) by providing cation-π interaction. Besides, the ethyl appendage of 3-ethylpiperidine occupies the hydrophobic region formed by F329.
Typical parallel π- π/cation-π stacking can be observed between tacrine and W82 (3.6 Å) ( Fig. 5C and D View Fig ) (Nachon et al., 2013). The amino group of tacrine is anchored to two water molecules, which forms a water-mediated bridge to other residues like D70, S79 and T120 (not shown). From the catalytic triad residues involved in the interaction with tacrine, hydrogen bonding between the aromatic nitrogen N 7 and the main chain carbonyl of H438 can be observed.
The overlay between 19 and tacrine is displayed in Fig. 6 View Fig . Most importantly, both ligands overlap within their aromatic regions that are implicated in the π- π/cation-π stackings with W82. Similarly, both ligands contact A328, W430 and Y332 by hydrophobic interactions, albeit differently. In the case of tacrine, the aromatic part of the molecule is engaged in these interactions; for 19, it is the 3-ethylpiperidine moiety that protrudes out to these residues. Besides the mutual amino acid residues involved in the interaction with both 19 and tacrine, 19 occupies some additional area of the h BuChE cavity given by the presence of the 3-ethylpiperidine moiety. This might become an area of interest for further structural changes to potentiate the inhibition ability of 19.
3. Conclusion
In conclusion, the detailed phytochemical study of aerial parts of V. minor led to the isolation of twenty-two known indole monoterpene alkaloids and one undescribed structure, named vincaminorudeine ( 9). Some previously reported literature NMR data had to be revised and updated. Bioassays focused on determining the cholinesterase activity related to AD revealed that some isolated indole alkaloids are promising h BuChE inhibitors. The most active structure, ()-2-ethyl-3[2-(3-ethylpiperidinyl)-ethyl]-1 H -indole ( 19), showed remarkable h BuChE, and POP inhibition potency. The possibility of blood-brain barrier penetration, enzyme kinetic analysis, and binding into the active site of h BuChE for 19 was explored as well. The previously described synthetic routes leading to the preparation of this compound and compelling results from bioassays open an exploration possibility for its use in therapy or to classify the compound as a leading structure for the development of novel analogues.
4. Experimental
4.1. General experimental procedures
NMR spectra were recorded for CDCl 3 solutions at laboratory temperature on a VNMR S500 (Varian) instrument operating at 500 MHz for proton nuclei and 125.7 MHz for carbon nuclei. Chemical shifts were recorded as δ values in parts per million (ppm) and were indirectly referenced to tetramethylsilane via the solvent signal (7.26 ppm for 1 H and 77.0 ppm for 13 C). The coupling constant ( J) is given in Hz, and the chemical shifts are reported in ppm. For unambiguous assignment of 1 H and 13 C signals, 2D NMR spectra (gCOSY, gHSQC, gHMBCAD, gH2BC, and NOESY) were obtained using standard parameter sets and pulse programs delivered by the manufacturer of the spectrometer. The MS (ESI) were measured on a Thermo Finnigan LCQDuo spectrometer. The HRMS (ESI) were obtained on a hybrid quadrupole-time-of-flight (Q- TOF) spectrometer (Waters Synapt G2Si coupled to a Waters Acquity I- Class UHPLC System). Optical rotation was measured on a P3000 polarimeter in either CHCl 3 or MeOH. Thin-layer chromatography (TLC) was carried out on Merck precoated silica gel F254 plates; the development solvents used were mixtures of cyclohexane (cHx), n -hexane ( n - Hx), toluene (To), chloroform (CHCl 3), acetone (Me 2 CO), diethylamine (Et 2 NH), acetonitrile (ACN), ethyl acetate (EtOAc), ethanol (EtOH), light petroleum (LPE), and trifluoroacetic acid (TFAA). All used chemicals and solvents were obtained either from Sigma Aldrich (the Czech Republic), Penta – Ing. ˇSvec (the Cech Republic), Lach-Ner (the Czech Republic), or VWR International ( France). Compounds were observed under UV light (254 and 366 nm), and alkaloids were detected by spraying with Dragendorff’ s reagent.
4.2. Plant material
The dried aerial parts of Vinca minor L. ( 60 kg) were purchased from the herbal dealer Megafyt s.r.o. (Vrane´nad Vltavou, the Czech Republic). The botanical verification was performed by prof. L. Opletal. The voucher specimen is deposited in the Department of Pharmaceutical Botany, Faculty of Pharmacy at Hradec Kr´alov´e, under code AL-350.
4.3. Extraction and isolation of alkaloids
Finely cut and dried aerial parts of V. minor were minced and sequentially extracted with 95% EtOH ( 500 g of material boiling with 3 L of EtOH for 30 min). The combined extracts were evaporated, and the dry residue was dissolved in 5% HCl. Hot distilled water was added, and the formed sediment (chlorophyll) was separated. After filtration, the extract was alkalized by an aqueous solution of NH 3 to pH 9–10. The precipitate was extracted with CHCl 3 (5 × 15 L). The combined CHCl 3 extracts were evaporated to dryness, yielding 454 g of dark brown alkaloidal residue. This residue was filtered through deactivated (by 6% of water) neutral aluminum oxide ( 4.5 kg), washing with CHCl 3 (9 L). The filtered extract was concentrated to give a dark brown residue ( 200 g).
This purified alkaloidal extract ( 200 g) was separated by column chromatography on deactivated (by 6% of water) neutral aluminum oxide ( 6 kg) eluted with LPE gradually enriched with CHCl 3 (95:5, 90:10, 87.5:12.5, 85:15, 80:20, 75:25, 70:30, 65:35, 60:40, 55:45, 50:50, 45:55, 40:60, 30:70, 15:85), then with CHCl 3 gradually enriched with EtOH (98:2, 98:4, 94:6, 92:8, 88:12, 80:20, 70:30, 60:40). In the end, the column was washed with pure EtOH. Each fraction was collected in 500 mL portions and was monitored by analytical TLC. Overall, over 500 fractions were collected; those with similar compositions were pooled together and evaporated to dryness, yielding 19 main fractions with an overall weight of 142 g. Based on TLC and GC-MS analysis, fraction No. 8 ( 27 g) appeared to be qualitatively and quantitatively the richest for alkaloids. Therefore, this fraction was chosen to be further processed and investigated.
In the pursuit of isolation of pure alkaloidal compounds, fraction No. 8 was fractionated by column chromatography on deactivated (by 10% of water) silica gel ( 1.5 kg), eluting firstly with a mixture of CHCl 3 and LPE (80:20), then with pure CHCl 3, and after that with CHCl 3 gradually enriched with EtOH (99:1, 98:2, 95:5, 90:10). The fractions were collected in 500 mL portions and monitored by analytical TLC sprayed with Dragendorff’ s reagent. Overall, over 100 fractions were collected; those with similar alkaloidal TLC profiles were pooled together and evaporated to dryness, providing 8 main alkaloid-rich fractions ( A–H).
Fraction A ( 0.59 g) was separated by preparative TLC (To–EtOAc–Et 2 NH, 40:15:1, × 2) to yield vincaminorine ( 1, 54.4 mg), recrystallized from a solution of CHCl 3 –EtOH 1:1, and vincaminoreine ( 2, 25.8 mg).
Fraction B ( 11.82 g) was dissolved in 5% HCl (pH 1) and filtered through Celite. The acidic solution of chlorides was extracted with Et 2 O (4 × 50 mL) and evaporated. The residue was alkalized with 10% Na 2 CO 3 (pH 9–10), and the white precipitate was extracted with Et 2 O (5 × 50 mL) and evaporated once again. The alkaloidal residue was filtered and washed with distilled water, yielding 5.21 g of ocher powder. Recrystallization from a mixture of EtOH and water 1:1 yielded eburnamonine ( 3, 37.5 mg).
Fraction C ( 1.36 g) was treated by preparative TLC ( n - Hx–EtOAc–Et 2 NH, 30:11:1, × 2) to give six alkaloidal subfractions ( C1–C6). Subfraction C1 was purified multiple times by preparative TLC in various mobile phases (mixtures of CHCl 3, EtOAc, ACN, and TFAA) to finally yield minovine ( 4, 12.6 mg). Subfraction C2 was joined with subfraction G1 and purified by preparative TLC to give 16-methoxyminovine ( 5, 27.3 mg). TLC separation of subfraction C 3 in various mobile phases (mixtures of cHx, LPE, and Et 2 NH) led to the isolation of a diastereoisomeric mixture 3:2 of vincatine ( 6, 98.1 mg). Subfraction C4, after treatment by preparative TLC (cHx–Et 2 NH, 95:5, × 2) and purification (CHCl 3 –ACN–TFAA, 40:10:0.1, × 1), afforded minovincine ( 7, 44.4 mg). Subfraction C5 was separated by preparative TLC (CHCl 3 –ACN–TFAA, 40:10:0.1, × 1) to yield 16-methoxyminovincine ( 8, 128.2 mg) and another compound, which after TLC purification (cHx–To–Et 2 NH, 65:30:5, × 2), afforded the undescribed alkaloid vincaminorudeine ( 9, 18.8 mg). Finally, subfraction C6, after treatment by preparative TLC (cHx–Et 2 NH, 95:5, × 2), yielded demethoxyalstonamide ( 10, 19.7 mg).
Preparative TLC (cHx–Et 2 NH, 95:5, × 2) was used to separate fraction D ( 3.13 g) into four alkaloidal subfractions ( D1–D4). Subfraction D1, after purification by preparative TLC in various mobile phases (mixtures of CHCl 3, ACN, TFAA, cHx, To, and Et 2 NH), yielded vincorine ( 11, 33.9 mg). Subfraction D2 contained eburnamonine ( 3, 9.8 mg). of subfraction H5 by TLC (EtOAc–MeOH–Et 2 NH, 40:10:2, × 1) achieved the isolation of raucubaine ( 23, 12.4 mg).
4.3.1. Vincaminorudeine (9)
Lightly brown crystals; [ α] D 27 + 27.8 (c 0.1, CHCl 3); for 1 H and 13 C NMR data see Table 1 View Table 1 ; HRMS m/z 371.2339 [M+ H] + (calc. for C 22 H 30 N 2 O 3 +, 371.2329); for HRMS and 1D and 2D NMR spectra see the Supplementary Data.
Subfraction D3, after further preparative TLC (cHx–EtOAc–Et 2 NH, 70:30:1, × 1), gave vincaminorudeine ( 9, 26.0 mg) and a mixture of two alkaloids, which after TLC separation (CHCl 3 –ACN–TFAA, 40:10:0.1, × 1) yielded 16-methoxyminovincine ( 8, 24.4 mg) and demethoxyalstonamide ( 10, 8.2 mg). Subfraction D4 was entirely composed of minovincinine ( 12, 499.4 mg).
Fraction E ( 1.02 g) was subjected to preparative TLC ( n- Hx–EtOAc–Et 2 NH, 30:11:1, × 2) to give three subfractions ( E1–E3). Subfraction E1 was purified by TLC (cHx–LPE–Et 2 NH, 80:20:4, × 3) to obtain aspidospermidine ( 13, 36.8 mg). Subfraction E2, merged with subfraction G3, was purified by repetitive TLC (To–Et 2 NH, 95:5, × 1; and To–Et 2 NH, 95:5, × 1) to afford 19-oxoeburnamonine ( 14, 10.0 mg). Separation of subfraction E3 (joined together with subfraction H3) by preparative TLC (CHCl 3 –ACN–TFAA, 40:10: 0.1, × 5) provided two alkaloidal zones, which after purification by further preparative TLC ( n- Hx–To–Et 2 NH, 45:45:10, × 2; and To–CHCl 3 –Et 2 NH, 70:25:5, × 2) afforded akuammicine ( 15, 8.9 mg) and tubotaiwine ( 16, 27.4 mg).
Fraction F ( 0.87 g), treated by repetitive preparative TLC ( n - Hx–EtOAc–Et 2 NH, 30:11:1, × 2), yielded raucubainine ( 17, 26.5 mg), which was recrystallized from EtOH.
Fraction G ( 0.41 g) was separated by preparative TLC to give three alkaloidal subfractions ( G1–G3). Subfraction G1 was joined with subfraction C2. Purification of subfraction G2 by TLC (To–EtOAc–Et 2 NH, 40:15:1, × 1) led to the acquisition of minovincine ( 7, 40.3 mg). Subfraction G3 was pooled together with subfraction E1.
Fraction H ( 2.38 g), after treatment by preparative TLC ( n -Hx: To–EtOAc–Et 2 NH, 20:10:11:1, × 3), afforded five main subfractions ( H1–H5). Subfraction H1 was divided by preparative TLC (cHx–Et 2 NH, 95:5, × 2) into two alkaloidal zones, which after purification by TLC (EtOAc–ACN–TFAA, 40:10:0.1, × 3; and n- Hx–EtOAc–Et 2 NH, 30:11:1, × 2) led to the isolation of aspidofractinine ( 18, 37.1 mg) and ()-2- ethyl-3[2-(3-ethylpiperidinyl)-ethyl]-1 H -indole ( 19, 27.5 mg). From subfraction H2, treated by multiple preparative TLC (cHx–Et 2 NH, 95:5, × 3; and cHx–LPE–Et 2 NH, 80:20:4, × 3), 14,15-dihydrovindolinine ( 20, 65.6 mg) was obtained. Subfraction H3 was eventually merged with subfraction E3. Subfraction H4 was separated by preparative TLC ( n- Hx–Et 2 NH, 90:10, × 3) into strictamine ( 21, 89.3 mg) and another alkaloidal zone, which after purification by TLC (CHCl 3 –ACN–TFAA, 40:10: 0.1, × 1) yielded 5-oxoaspidofractinine ( 22, 3.8 mg). Purification
4.4. Biological assays
4.4.1. Inhibition of hAChE, hBuChE
The activity of isolated alkaloids for the inhibition of human cholinesterases was assessed using the modified version of Ellman’ s method (Ellman et al., 1961), described recently (Al Mamun et al., 2020). The detailed description of the assay can be found in the Supplementary Data.
4.4.2. Kinetic study of hBuChE inhibition
The pharmacokinetic study of the most active substance was evaluated with the same procedure as described recently (Hostalkova et al., 2019). The details can be found in the Supplementary Data.
4.4.3. Inhibition of POP
For this assay, the same method was used as previously described (Al Mamun et al., 2020). The detailed protocol can be found in the Supplementary Data.
4.4.4. Inhibition of GSK-3 β
The method for this biological test was performed according to the earlier study (Hulcova et al., 2018). Details of the procedure can be found in the Supplementary Data.
4.4.5. CNS penetration: In vitro parallel artificial membrane permeability assay (PAMPA)-blood brain barrier (BBB)
The same procedure was used as in our previous report (Cahlikova et al., 2015; Panek et al., 2017). Details of the study are available in the Supplementary Data.
4.5. Docking study
Molecular docking was used for binding poses calculations. The 3D structure ligands were built by OpenBabel, v. 2.3.2 (O’ Boyle et al., 2011) and optimized by Avogadro, v. 1.2.0 using the force fields GAFF (Hanwell et al., 2012). They were converted into pdbqt-format by OpenBabel, v. 2.3.2. The h BuChE structure was gained from the RCSB database (PDB ID: 4BDS, resolution 2.10 Å) and prepared for docking by the function DockPrep of the software Chimera, v. 1.14 (Pettersen et al., 2004) and by MGLTools, v. 1.5.4 (Morris et al., 2009). The docking calculation was made by Vina, v. 1.1.2 as semi-flexible with flexible ligand and rigid receptor (Trott and Olson, 2010).
The docking pose of 19 was improved by MD simulation. The receptor structure was prepared using the software Chimera. The bestscored docking pose was taken as the initial for MD. The force-field parameters for ligands were assessed by Antechamber (Wang et al., 2006), v. 20.0 using General Amber force-field 2 (Wang et al., 2004). MD simulation was carried out by Gromacs, v. 2018.1 (Abraham et al., 2015). The complex receptor-ligand was solvated in the periodic water box using the TIP3P model (Mark and Nilsson, 2001). The system was neutralized by adding Na + and Cl ions to a concentration of 10 nM. The system energy was minimalized and equilibrated in a 100-ps isothermal-isochoric NVT and then a 100-ps isothermal-isobaric NPT phase. Then, a 10-ns MD simulation was run at a temperature of 300 K. The molecular docking and MD results were 3D visualized by the PyMOL Molecular Graphics System, Version 2.4.1, Schr¨odinger, LLC.
| N |
Nanjing University |
| MS |
Herbarium Messanaensis, Università di Messina |
| UV |
Departamento de Biologia de la Universidad del Valle |
| H |
University of Helsinki |
| NMR |
Natuurhistorisch Museum |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |