Chagasia Cruz
|
publication ID |
https://doi.org/10.5281/zenodo.189830 |
|
DOI |
https://doi.org/10.5281/zenodo.6222449 |
|
persistent identifier |
https://treatment.plazi.org/id/7E4C879A-AD57-F340-1380-FAB839AB7786 |
|
treatment provided by |
Plazi |
|
scientific name |
Chagasia Cruz |
| status |
|
Genus Chagasia Cruz View in CoL View at ENA
Type species: Chagasia neivae Cruz (monotypy).
Chagasia Cruz, 1906: 199 View in CoL (new genus), haplotype: neivae Cruz (1906: 199) View in CoL . Edwards, 1911: 141 (to subgeneric status in Anopheles View in CoL , but later abandoned); Christophers, 1924: 5, 7 (to subgeneric status in Anopheles View in CoL ); Root, 1923: 267, Root, 1927: 471 (generic status reinstated).
Pyretophorus in part of Lutz, 1904, in Bourroul, 1904: 64; Blanchard, 1905: 623.
Chagasia View in CoL of Peryassú, 1908: 33, 41, 61, 121–125; Theobald, 1907: 122 –124; Theobald, 1910: 3, 75, 77, 79; Surcouf & Gonzalez-Rincones, 1911: 37, 41–44; Brunetti, 1914: 22, 33, 34, 57; Peryassú, 1921a: 71; Peryassú, 1923: 63; Root, 1923: 267, 270; Root, 1927: 471 –474; Shannon & del Ponte, 1928: 36, 38, 61; Edwards, 1930: 287; Shannon, 1931: 131, 135, 136, 152–153; Edwards, 1932: 29, 31–32; Martini, 1935: 4, 11, 14; Pinto, 1939: 304; Gabaldon et al., 1940: 57; Kumm et al., 1940: 413, 414, 419; Vargas, 1940: 191; Komp, 1941: 89, 90, 91, 92, 94, 96; Floch & Abonnenc, 1942: 1; Simmons & Aitkin, 1942: 38, 39 40, 41, 46, 47, 54; Gast Galvis, 1943: 6, 7, 8–9, 19; Komp, 1942: 38, 79, 131, 166, 177, 180; Russell et al., 1943: 6, 7, 24, 30, 35, 39, 42; Leví-Castillo, 1945: 2, 13, 15–16. pl. XI; Pelaez, 1945: 70, 71; Causey et al., 1946: 25; Deane, L.M. et al., 1946: 8; Deane, M.P. et al., 1946a: 40; Deane, M.P. et al., 1946b: 360; Deane, L.M. et al., 1948: 831; Rachou, 1948: 13; Vargas & Martinez Palacios 1950: 2, 17, 42, 43, 47, 50, 54, 61; Floch & Abonnenc, 1951: 8, 9, 10, 21, 22, 23, 27; Ross, 1951: 129; Levi-Castillo, 1951: 77, 79; Lane, 1953: 138 –147; Horsfall, 1955: 41; Vargas & Martinez Palacios, 1956: 10, 16, 20, 41, 44, 45, 48, 52, 55; Senevet, 1958: 6, 7–9; Stone et al., 1959: 6, 10; Cova-Garcia, 1961: 167, 168, 173–174, 178; Rodriguez, 1961: 217, 218, 222; Belkin, 1962: 117, 123, 124, 125, 126, Fig. 37; Forattini, 1962: 180, 285, 303, 304, 305, 306, 467, 468; García & Ronderos, 1962: 124, 125, 139; Forattini et al., 1970: 20; Mattingly, 1971: 4, 9, 15, 21, 29; Cova Garcia & Sutil O., 1976: 15 –16; Cova Garcia & Sutil O., 1977: 7 –8; Knight & Stone, 1977: 2, 67–68; Harbach & Knight 1980: 114, 131, 140; Darsie, 1985: 158, 172, 193, 221, 237; Clark-Gil & Darsie, 1990: 167, 183, 206, 218; Forattini, 1996: 232, 233; Guimarães, 1997: 1, 2, 29–30; Harbach & Sandlant, 1997; Harbach & Kitching, 1998: 335, 336, 342, 343, 346, 349, 350, 352, 353, 359, 360, 367; Rueda et al., 1998; Reinert, 1999: 77, 81; Sallum et al., 2000: 745, 748, 769, 770, 771, 774; Krzywinski et al., 2001a: 479, 480, 484, 486; Krzywinski et al., 2001b: 540, 542, 552, 553; Forattini, 2002: 36, 191, 192, 193–195, 236–241, 802; Huang, 2002: 2, 25; Sallum et al., 2002: 361, 362, 367, 369, 370, 374, 375, 376; Krzywinski & Besansky, 2003: 115, 116, 117; Harbach & Kitching, 2005: 345, 346, 347, 351, 352, 355, 362, 364; González & Carrejo, 2007: 11, 32, 35, 36; Harbach, 2007: 594, 596, 600, 601, 606, 608, 609, 610, 611, 612, 628.
Anopheles ( Chagasia) View in CoL of Edwards, 1911: 141; Dyar, 1918: 142, 149; Root, 1922: 388; Christophers, 1924: 15, 77, 78; Bonne & Bonne-Wepster, 1925: 497, 543–546; Dyar, 1928: 431 –433; Komp, 1936: 66.
Diagnosis. The adults of Chagasia are similar to those of Anopheles , but the resting posture is like culicine mosquitoes with the head and abdomen at angles to the thorax and the scutellum is tri-lobed with setae in three distinct groups. The wings are principally dark-scaled or have a mixture of dark and pale scales. Eggs have a circumferential covering of longitudinal floats and the micropylar apparatus is borne dorsally at the anterior end. Larvae have three pairs of exceptionally long broom-like dorsal cranial setae (setae 2,4,6-C) that project forward from the anterior margin of the head, they bear uniquely shaped palmate setae (seta 1) on abdominal segments III–V and the spiracular apparatus has a long filamentous anterior median process and a fringe-like row of setae on either side. Pupae have a strong dorsal spine (seta 2) on segments III to VII in addition to the strong lateral spine (seta 9-V–VIII) that is usually present in anopheline mosquitoes. The apical seta of the pupal paddle (seta 1-Pa) is also stout and spine-like.
Females. In general as in Anopheles except for the following striking differences. Head: Eyes narrowly separated above antennae; dorsum with narrow elongate forked scales and broad falcate scales from posterior margin (occiput) confluent with dorsolateral line and a wide median band of similar scales on vertex, ocular line and interocular space, space between median band and dorsolateral line without scales; postgena with scales anteriorly; clypeus bare. Antenna slightly shorter than proboscis; pedicel with scales dorsally; flagellum bare ventrally, flagellar whorls reduced to relatively few short setae at apex of flagellomeres, flagellomere 2 short, about half length of other flagellomeres, apices of flagellomeres 1–9 with dorsal patch of dark scales. Proboscis about same length as forefemur, dorsal surface narrowly without scales, sides and venter entirely dark-scaled, scales semi-erect to near labella. Maxillary palpus slightly longer than proboscis, comprised of 5 palpomeres, ventral surface narrowly without scales, sides and dorsum with semi-erect dark scales (very shaggy) and few pale scales dorsally at apices of palpomeres 2–4. Cibarial armature formed of 3 large teeth between the lateral flanges ( Romeo Viamonte & Castro, 1951). Thorax: Scutum with distinct lines of decumbent generally white spatulate scales along acrostichal and anterior dorsocentral setae [posterior acrostichal scales dark in Ch. ablusa Harbach , n. sp., Ch. fajardi (Lutz) and Ch. rozeboomi Causey, Deane & Deane ] and margins of scutal fossa and prescutellar area; posterior dorsocentral area with decumbent to semierect dark spatulate scales; antealar and supraalar areas with long outstanding dark truncate spatulate scales. Scutellum trilobed with median and lateral clusters of setae and narrow white spatulate scales on median lobe extending on either side to setae of lateral lobes. Mesopostnotum bare; antepronotum, postpronotum, anterior area of paratergite, upper proepisternum, upper and lower areas of mesokatepisternum and upper area of mesepimeron with narrow pale spatulate scales; setae present on these areas, except paratergite, as well as on prespiracular and prealar areas; lower mesepimeron, mesomeron and metameron bare; prealar area not separated by suture from mesokatepisternum; mesomeron relatively large, its upper edge above base of hindcoxa. Wing: Membrane with distinct microtrichia; spatulate scales of veins relatively narrow to broad, scales all dark, almost entirely dark or mixture of pale and dark scales; cell R2 longer than vein R2+3; vein R s (contrary to Harbach & Kitching, 1998) apparently without basal spur; vein 1A ending well beyond furcation of mcu and CuA; vein R without dorsal remigial setae; subcosta without distinct setae ventrally at base; alula bare; upper calypter with complete row of marginal setae. Legs: Very long and slender; femora with speckles and blotches or spots of pale scales and narrow apical pale fringe, sometimes with ill-defined preapical pale patch on anterior surface; tibiae with dorsoanterior blotches or spots of pale scales, without tibial setae; tarsi with pale bands, hindtarsomere 1 usually with 5 or 6 pale bands (range = 4–7); all ungues simple, fore- and midungues noticeably larger than hindungues; pulvilli not developed. Abdomen: Terga and sterna without scales, densely setose; laterotergite bare. Genitalia: Not studied; one spermathecal capsule.
Males. Similar to females except for obvious sexual differences; other differences include the following. Head: Antennal pedicel strongly swollen and much larger than in females; flagellum strongly verticillate. Maxillary palpus slightly longer than proboscis, with 5 palpomeres, palpomeres 4 and 5 not conspicuously swollen. Legs: Ungues of fore- and midlegs large, anterior unguis slightly larger, with 2 teeth, one at base and one at midlength, posterior unguis with single tooth at base; hindungues as in females. Genitalia: In general as in Anopheles except for the following distinctive differences, which are shown in Fig. 1 View FIGURE 1 C. Segment IX reduced, tergum and sternum fused, tergum IX bi-lobed, densely spiculate, with prominent setae, sternum IX without setae; gonocoxite simple, without scales, with dorsomesal prominence bearing specialised stout spine-like setae; gonostylus long, slender, with row of minute setae along sternomesal margin and short apical flattened claw; claspette simple, lobe-like, densely spiculate, with or without setae; aedeagus long, more or less cylindrical, without apical leaflets; proctiger membranous, paraprocts weakly sclerotised; cercal setae absent.
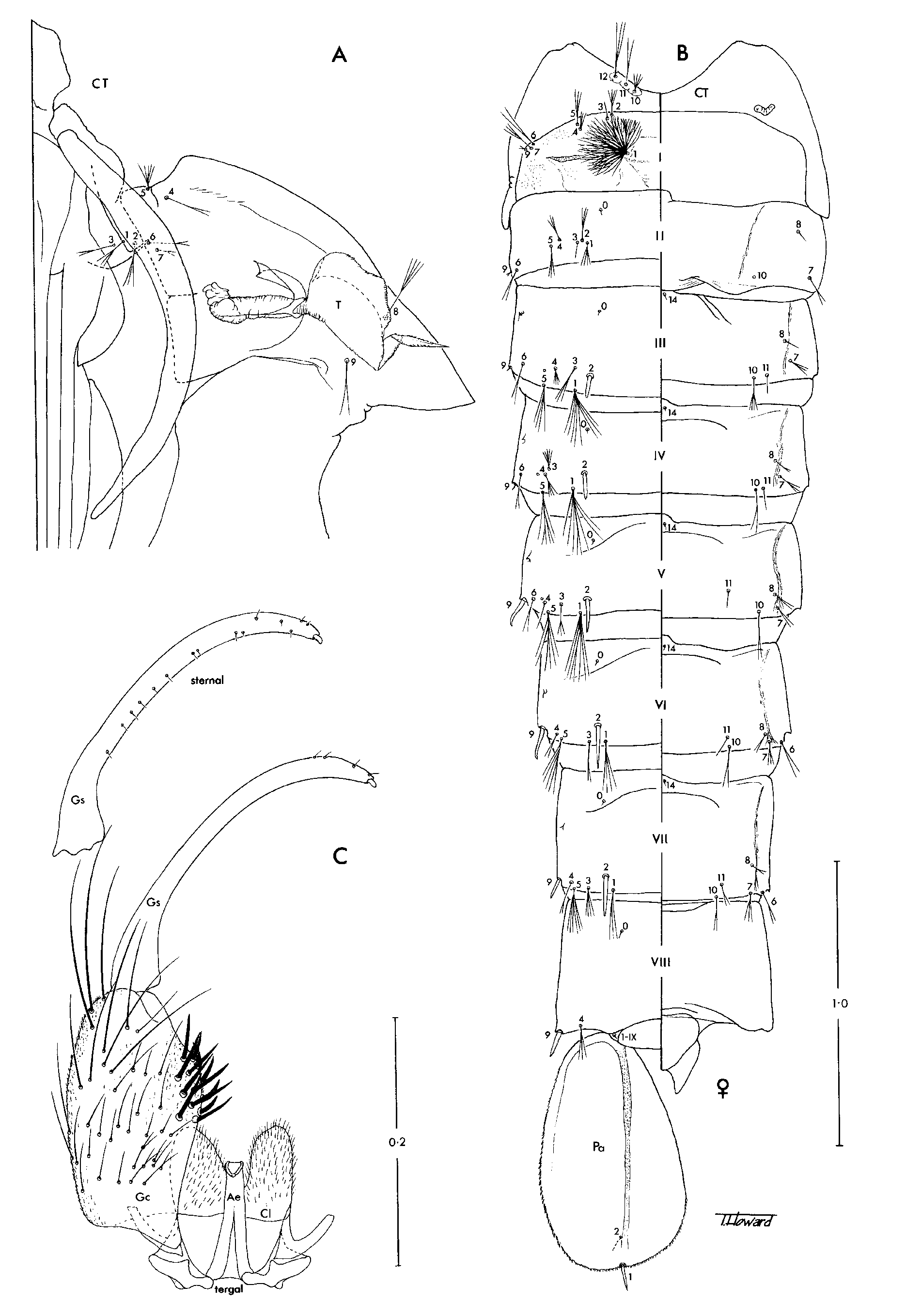
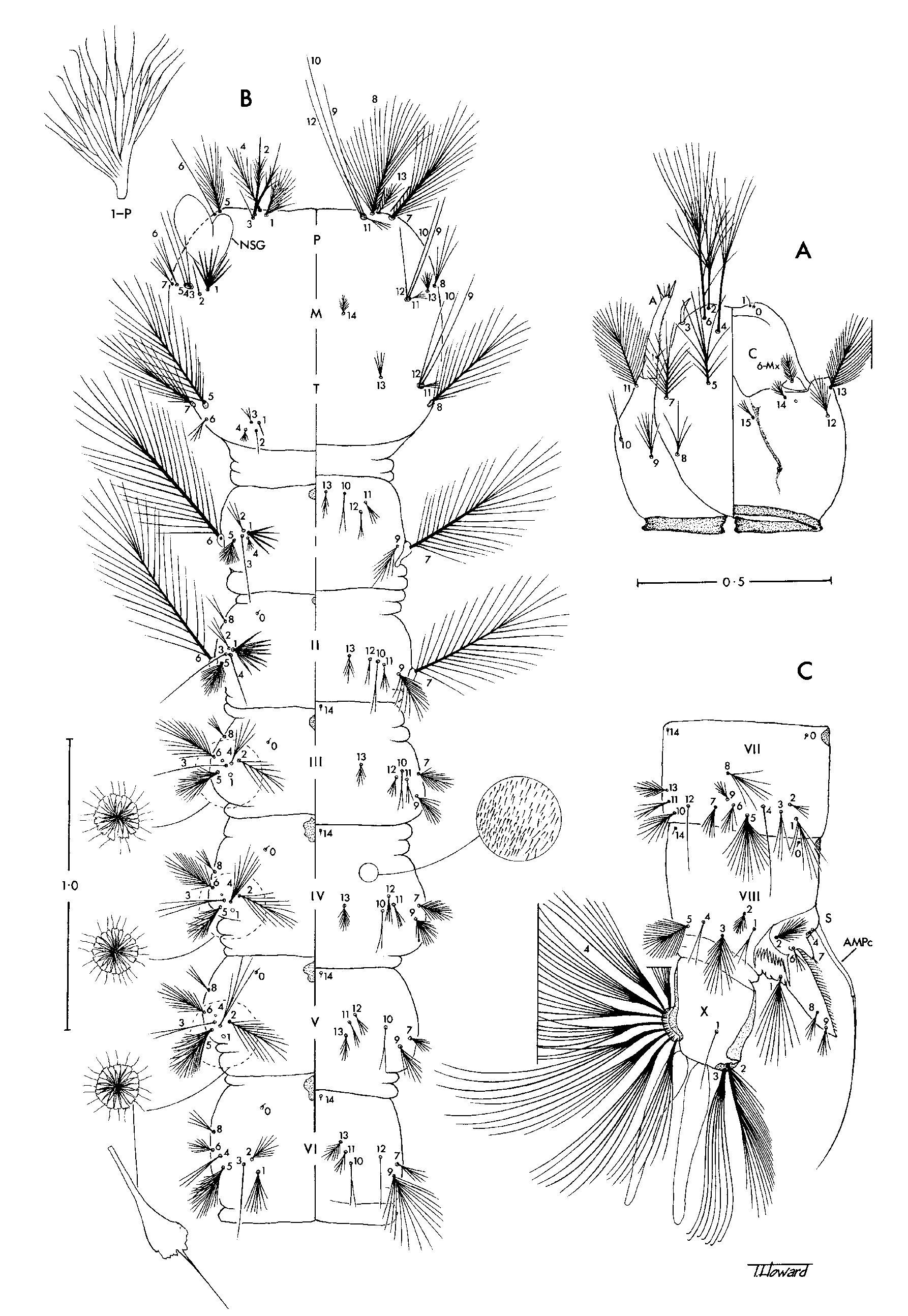
Pupae. In general as in Anopheles ; known in detail only for Ch. bonneae ( Fig. 1 View FIGURE 1 A,B). Cephalothorax: Dorsal apotome evenly sclerotised, undivided; middorsal ridge well developed; all setae present, rather short. Trumpet: Laticorn, strongly flared and deeply divided to near base, without tragus; tracheoid area present; placed on basal tubercle. Abdomen: Segments III–VII with ventral fold lines; tergum IX distinct, not fused with tergum VIII; seta 1-I strongly developed, dendritic; setae 1-II–VII and 5-II–VII similarly developed, branched; seta 2-III–VII single, stout, spine-like, 2-III–V inserted mesad of seta 1, 2 -VI,VII inserted lateral of seta 1; seta 6-II–V inserted anterodorsal and mesal to seta 9, 6 -VI,VII inserted posteroventral to seta 9; seta 9- I shorter than seta 6-I; seta 9-II–VIII single, stout, spine-like, 9-VI,VII inserted slightly anterior to caudolateral angle of segment, 9-VIII inserted at caudolateral angle; seta 0-VII inserted on anterior 0.5 of tergum (as in culicine mosquitoes); seta 4-VIII inserted mesad of seta 9; seta 14-VIII usually absent, very weak and inserted near midline when present; seta 1-IX present; 1-X absent. Genital lobe: Cercus well developed in female, projecting beyond apex of genital lobe; genital lobe of male slightly tapered distally, apex broadly rounded. Paddle: Longer than broad; external buttress more or less distinct; midrib long, distinct to near tip of paddle; outer part broader than inner part; outer margin and distal part of inner margin with minute spines; setae 1,2-Pa present, 1-Pa stout, spine-like, inserted at apex; 2-Pa removed cephalad from apical margin on ventral surface.
Larvae, fourth-instars. In general as in Anopheles ; as exemplified by Ch. bonneae ( Fig. 2 View FIGURE 2 ). Head: Width slightly greater than length; collar wider than distance between antennae; posterior tentorial pit (PTP) at considerable distance from caudal border; hypostomal suture complete, extending slightly caudad of PTP; cephalic border of labiogula produced in front; hypostomal sclerite (“cardo”) triangular, width greater than length; seta 1-C small, arising ventrally immediately mesad of seta 0-C; setae 2,4,6-C strongly developed, inserted far forward with 4-C more posterior than 6-C; broom-like with long stem and long distal branches, about 0.75 length of head capsule; seta 3-C stout, spine-like, inserted at margin of cranium laterad of 6-C; setae 5,7-C rather weakly plumose, 5-C inserted more or less on level of base of antenna, 7-C inserted posterior to this level; seta 13-C strongly plumose, large, inserted more or less on level with seta 11-C. Antenna: Shorter than head capsule, ventral surface spiculate; seta 1-A small, inserted dorsomesally in basal half; seta 2-A inserted subapically; seta 4-A short, single. Thorax: Lateral and ventral surfaces densely spiculate; seta 0-P apparently absent; seta 1-P asymmetrically branched, lanceolate branches arise on one side of main stem; seta 2-P with 2 stout divergent branches; seta 4-P inserted anterior to 2,3-P; Nuttal and Shipley’s organ caudad of setae 5,6-P; setae 7,8-P and 5,7,8-T long, strongly plumose; seta 9-P on tubercle with setae 10–12-P (contrary to Belkin, 1962: 124, Sallum et al., 2000 and Harbach & Kitching, 2005); seta 14-P absent; seta 1-M usually with lanceolate branches; setae 3–5-M on common tubercle; seta 8-T inserted posterolaterad of setae 9–12-T. Abdomen: Lateral and ventral surfaces densely spiculate; single tergal plate anteriorly on segments I–VII; seta 0-II–VII more mesal than other dorsal setae; seta 1-I,II,VI,VII not palmate, 1-I,II usually with lanceolate branches, 1-VI,VII with normal branches; seta 1-III–V uniquely palmate, branches with distally expanded blade and hair-like apical filament; seta 2-I,II,VI inserted anterolateral and 2- III–V inserted anteromesal to seta 1; setae 6,7-I,II long and strongly plumose (as in other anophelines); setae 2,5,6,7,9-III–VI short and plumose. Spiracular apparatus (see Harbach & Knight, 1980: Fig. 64d): Anterior median process very long, filamentous; posterolateral spiracular lobe with fringe of setae on outer margins (as in dixid larvae). Segment X: Saddle a small dorsal sclerite; seta 1-X inserted on integument adjacent to margin of saddle, single; setae 2,3-X strongly developed, 2-X distinctly asymmetrically branched, shorter than 3-X, 3-X hooked at apex; seta 4-X (ventral brush) very strongly developed, with 9 pairs of setae.
Eggs. Surface almost entirely covered by multiple longitudinal floats with numerous transverse ridges; without large areas of outer chorionic cells, however limited areas between floats at anterior and posterior ends of eggs of Ch. fajardi bear cells with floors perforated by pores ( Linley & Milstrey, 1995); anterior end abruptly tapered, apex with small area bound by collar and bearing variable number of lobed tubercles; posterior end more gradually tapered, apex with larger area bound by collar, area with variable number of lobed tubercles around periphery, floor of area with few chorionic cells; lobed tubercles widely separated, generally thin and not swollen apically, less compact than those of Anopheles eggs; micropylar apparatus borne dorsally near collar at anterior end, bound by narrow ridge-like collar, surface of collar nodular, tending to be flat posteriorly and elevated anteriorly, disk fairly smooth with central radial depressions surrounding inconspicuous micropyle.
Discussion. Genus Chagasia is a small homogenous group of species that exhibit characteristics of both subfamilies Anophelinae and Culicinae , but are obviously more closely allied with anopheline taxa based on overall morphology of the immature stages. Certain features of the adults, especially the scaling of the scutum and wings, bear a resemblance to the adults of genus Aedeomyia , which based on morphology and distribution appears to be a primitive group of subfamily Culicinae ( Belkin, 1962; Harbach & Kitching, 1998). The male proctiger of Aedeomyia species, like that of Chagasia and other anophelines, as well as Uranotaenia and a few aedines, is largely unsclerotized and lacks cercal setae. Whereas Chagasia are mainly found in tropical areas of the Neotropical Region, species of Aedeomyia principally occur in tropical areas of the Southern Hemisphere. In as much as the analysis of Harbach & Kitching (1998) indicated that Aedeomyia is a cladistically basal group of subfamily Culicinae , it is possible that Chagasia and Aedeomyia could have been early offshoots of an ancestral lineage in the Southern Hemisphere. It is also interesting to note that Chagasia are the only species of Culicidae that bear a fringe of setae on the posterolateral lobes of the larval spiracular apparatus in common with species of family Dixidae .
Bionomics. Chagasia larvae are usually found in shaded streams among the roots of trees and in grassy margins or dead leaves and other debris. They sometimes occur in clear rock-pools along shaded streams. Adults remain in vegetation near the larval habitats or enter nearby forest canopy. Females bite during the day and night, but seldom feed on humans. Species of Chagasia are not known to transmit pathogens of human diseases.
Distribution. Chagasia are Neotropical mosquitoes. The distribution of Ch. bathana extends from Peru, Colombia and Venezuela through Central America into southern Mexico. The other species are restricted to South America. Country records for each species are listed below.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Anophelinae |
Chagasia Cruz
| Harbach, Ralph E. & Howard, Theresa M. 2009 |
Anopheles ( Chagasia )
| Komp 1936: 66 |
| Dyar 1928: 431 |
| Bonne 1925: 497 |
| Christophers 1924: 15 |
| Root 1922: 388 |
| Dyar 1918: 142 |
| Edwards 1911: 141 |
Chagasia
| Gonzalez 2007: 11 |
| Harbach 2007: 594 |
| Harbach 2005: 345 |
| Krzywinski 2003: 115 |
| Forattini 2002: 36 |
| Sallum 2002: 361 |
| Krzywinski 2001: 479 |
| Krzywinski 2001: 540 |
| Sallum 2000: 745 |
| Reinert 1999: 77 |
| Harbach 1998: 335 |
| Guimaraes 1997: 1 |
| Forattini 1996: 232 |
| Darsie 1985: 158 |
| Harbach 1980: 114 |
| Cova 1977: 7 |
| Knight 1977: 2 |
| Cova 1976: 15 |
| Mattingly 1971: 4 |
| Forattini 1970: 20 |
| Belkin 1962: 117 |
| Forattini 1962: 180 |
| Garcia 1962: 124 |
| Cova-Garcia 1961: 167 |
| Rodriguez 1961: 217 |
| Stone 1959: 6 |
| Senevet 1958: 6 |
| Vargas 1956: 10 |
| Horsfall 1955: 41 |
| Lane 1953: 138 |
| Floch 1951: 8 |
| Ross 1951: 129 |
| Levi-Castillo 1951: 77 |
| Vargas 1950: 2 |
| Deane 1948: 831 |
| Rachou 1948: 13 |
| Deane 1946: 40 |
| Deane 1946: 360 |
| Levi-Castillo 1945: 2 |
| Pelaez 1945: 70 |
| Gast 1943: 6 |
| Russell 1943: 6 |
| Floch 1942: 1 |
| Simmons 1942: 38 |
| Komp 1942: 38 |
| Komp 1941: 89 |
| Gabaldon 1940: 57 |
| Kumm 1940: 413 |
| Vargas 1940: 191 |
| Pinto 1939: 304 |
| Martini 1935: 4 |
| Edwards 1932: 29 |
| Shannon 1931: 131 |
| Edwards 1930: 287 |
| Root 1927: 471 |
| Peryassu 1923: 63 |
| Root 1923: 267 |
| Peryassu 1921: 71 |
| Brunetti 1914: 22 |
| Surcouf 1911: 37 |
| Theobald 1910: 3 |
| Peryassu 1908: 33 |
| Theobald 1907: 122 |
Chagasia
| Root 1927: 471 |
| Christophers 1924: 5 |
| Root 1923: 267 |
| Edwards 1911: 141 |
| Cruz 1906: 199 |
| Cruz 1906: 199 |
Pyretophorus
| Blanchard 1905: 623 |
| Bourroul 1904: 64 |