Gromphas inermis Harold, 1869
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3722.4.2 |
|
publication LSID |
lsid:zoobank.org:pub:20D125E7-8CC0-4942-8AF9-75EA31EBBD53 |
|
DOI |
https://doi.org/10.5281/zenodo.5668597 |
|
persistent identifier |
https://treatment.plazi.org/id/707787C6-5963-6261-FF0A-238D330E9A75 |
|
treatment provided by |
Plazi |
|
scientific name |
Gromphas inermis Harold, 1869 |
| status |
|
5. Gromphas inermis Harold, 1869 View in CoL
Fig. 15 View FIGURES 13 – 15. 13 , 20, 23, 28, 32, 40, 43, 50, 55, 59, 65–66
Gromphas inermis Harold, 1869b: 62, 1869a: 1016 ; Bates 1870: 175; Burmeister 1874: 130; Preudhomme-de-Borre 1886: 105; Judulien 1899: 375; Gahan & Arrow 1903: 246; Heyne & Taschenberg 1908: 64; Tremoleras 1910: 25; Bruch 1911: 189; Gillet 1911: 80; d’Olsoufieff 1924: 59; Blackwelder 1944: 208; Barattini & Saenz, 1960: 25, 1964: 179; Halffter & Matthews 1966: 131; Martínez 1989: 67; Tylianakis & Dimaki 2006: 291.
Gromphas lacordairii Burmeister, 1874: 130 (cited as “ G. lacordairei Dejean ” or “ G. lacordairei Brullé ”); Lacordaire 1876: 10, plate 27 Fig. 4; Tremoleras 1910: 25; Bruch 1911: 189; Gillet 1911: 80; Fabre 1919: 244; d’Olsoufieff 1924: 20, 59, 139, plate II Fig. 2, plate IX; Manter 1928: 345; Pessoa & Lane 1941: 472; Blackwelder 1944: 208; Lange 1947: 313; Lima 1953: 62; Ruffinelli & Carbonell 1953: 25; Janssens 1954: plate II; Martínez 1959: 95; Barattini & Saenz 1960: 25, 1964: 179; Halffter & Matthews 1966: 131, 225; Halffter & Edmonds 1982: 84, 86; Martínez, 1989: 67; Flechmann et al. 1995: 267; Flechmann & Rodrigues 1995: 304; Louzada et al. 1996: 164; Monteresino et al. 1996: 109; Walsh & Gandolfo 1996: 582, 583 Fig. 1E, 285–287; Walsh & Cordo 1997: 194; Martínez & Cruz 1999: 805; Koller et al. 1999: 405, 2007: 85; Vazde-Mello 2000: 193; Morelli et al. 2002: 54; Hamel-Leigue 2006: 6, 2009: 49, 2013: 281; Damborsky et al. 2008: 149; Sánchez & Genise 2008: 49; Vieira et al. 2008: 722; Almeida & Mise 2009: 238; Silva et al. 2009: 36; Audino et al. 2011: 125; González-Hernández & Navarrete-Heredia 2011: 479; Silva 2011: 552; Vaz-de-Mello et al. 2011: 59, Fig. 95; Figueroa et al. 2012: 2.
Gromphas lacordairei bipunctata d’Olsoufieff, 1924: 59 (cited as “ var. bipunctata ”); Vaz-de-Mello 2000: 193. New synonymy
Type specimens:— G. inermis Harold, 1869 : Lectotype: here designated, male (handwritten “Buenos Aires”, Harold’s handwritten “ inermis Harold ”, printed on white paper bordered in black “Ex musaeo E. Harold”, LECTOTYPE ”, “ LECTOTYPE Gromphas inermis Har. Vaz-de-Mello, 2013”), MNHN, ex col. Oberthür (examined). Paralectotype: Not located at MNHN unknown to us.— G. lacordairei bipunctata d’Olsoufieff, 1924: Lectotype: here designated, male (bordered in black “ Brésil, Prov. Matto Grosso, P. Germain, 1886”, “ LECTOTYPE ”, “ LECTOTYPE Gromphas lacordairei var. bipunctata Ols. Vaz-de-Mello, 2013”), MNHN, ex col. Oberthür (examined). Paralectotype: Not located at MNHN; unknown to us.
FIGURES 16–20. Lateral views. 16) Gromphas aeruginosa . 17) G. lemoinei . 18) G. amazonica . 19) G. dichroa . 20: G. inermis . 20a) Major specimen (the white arrow indicates “pronotal hump” and blue arrow indicates elevation of sutural margin of elytra). 20b) Medium-sized specimen. 20c) Minor specimen. 20d) Lateral view of row of tubercles on ventral carina of protibia (indicated by arrow). Scale = 5 mm.
Type locality: G. inermis : Buenos Aires, Argentina (type locality of the lectotype, according Article 76.2 of the Code [International Commission on Zoological Nomenclature 1999]). G. lacordairei bipunctata : Mato Grosso, Brazil (type locality of the lectotype).
Redescription: Color: Anterior region of clypeus black; remainder of dorsum with metallic reflections and color ranging from black ( Fig. 15 View FIGURES 13 – 15. 13 c) to dark green ( Fig. 15 View FIGURES 13 – 15. 13 b) or entirely copper with green reflections ( Fig. 15 View FIGURES 13 – 15. 13 a). Metasternum with distinct color, usually bright dark blue, blue with green reflections, or shiny green. Pygidium green or blue.
Head: Margin of clypeus with four lobes ( Fig. 28 View FIGURES 24 – 28 ); apical margin folded upwardly, especially two median lobes. Genae and frons completely granulate, including region adjacent to eyes ( Fig. 28 View FIGURES 24 – 28 ). Cephalic projection raised in a carina with emarginate apex in major specimens ( Fig. 32 View FIGURES 29 – 33 ), and only slightly projecting with rounded apex in minor specimens.
Thorax: Pronotum globular, in larger specimens, with evident anterior swelling (“pronotal hump”, Fig. 20a, white arrow); lateral region and center with dense granulation (Figs. 20a–d); posterior region smooth ( Figs. 15 View FIGURES 13 – 15. 13 a–c). Longitudinal midline of glossy and smooth tegument absent or, rarely, very tenuous; posterior fossae usually absent, represented in some specimens by two very shallow, faint impressions ( Fig. 43 View FIGURES 34 – 48. 34 ). Posterior margin projected at middle ( Fig. 15 View FIGURES 13 – 15. 13 a–c).
Mesosternum with dense pilosity ( Fig. 52 View FIGURES 49 – 52 ). Metasternum with very dense punctation in center. Anteromedian angle of metasternum convex and with globose apex ( Fig. 50 View FIGURES 49 – 52 ); area in front of anteromedian angle with evident setae (Figs. 20, 50).
Legs: Protibiae narrower in males than in females ( Fig. 23 View FIGURES 21 – 23 ); in ventral view, longitudinal carina with a row of tubercles on basal one-half in males (Figs. 20d, 23b) and simple in females ( Fig. 23 View FIGURES 21 – 23 a). Protibial spur with apex strongly expanded and curved downward ( Figs. 21, 23 View FIGURES 21 – 23 , 45 View FIGURES 34 – 48. 34 ); inner apical angle of protibiae with a tuft of setae longer in males than in females ( Fig. 23 View FIGURES 21 – 23 ). Apical protarsomere short, without spiniform projection ( Fig. 46 View FIGURES 34 – 48. 34 ). Mesotarsi and metatarsi not particularly enlarged and with apical tarsomeres only slightly curved apically ( Fig. 48 View FIGURES 34 – 48. 34 ). Metatibiae very broad and robust ( Fig. 41 View FIGURES 34 – 48. 34 ). Metatibial spur with apex straight ( Fig. 40 View FIGURES 34 – 48. 34 ).
Elytra: Striae very fine and simple, not carinulate. Sutural margin densely punctate and clearly raised in its apical half in major specimens (Fig. 20a, blue arrow); in basal third or basal half, sheen and punctation of sutural margin extend onto first or second interstriae (easily seen in Fig. 15 View FIGURES 13 – 15. 13 b).
Abdomen: Pygidium flat, not margined basally ( Fig. 36 View FIGURES 34 – 48. 34 ). Groove of propygidium extending to base of pygidium. Abdominal sternites densely punctate.
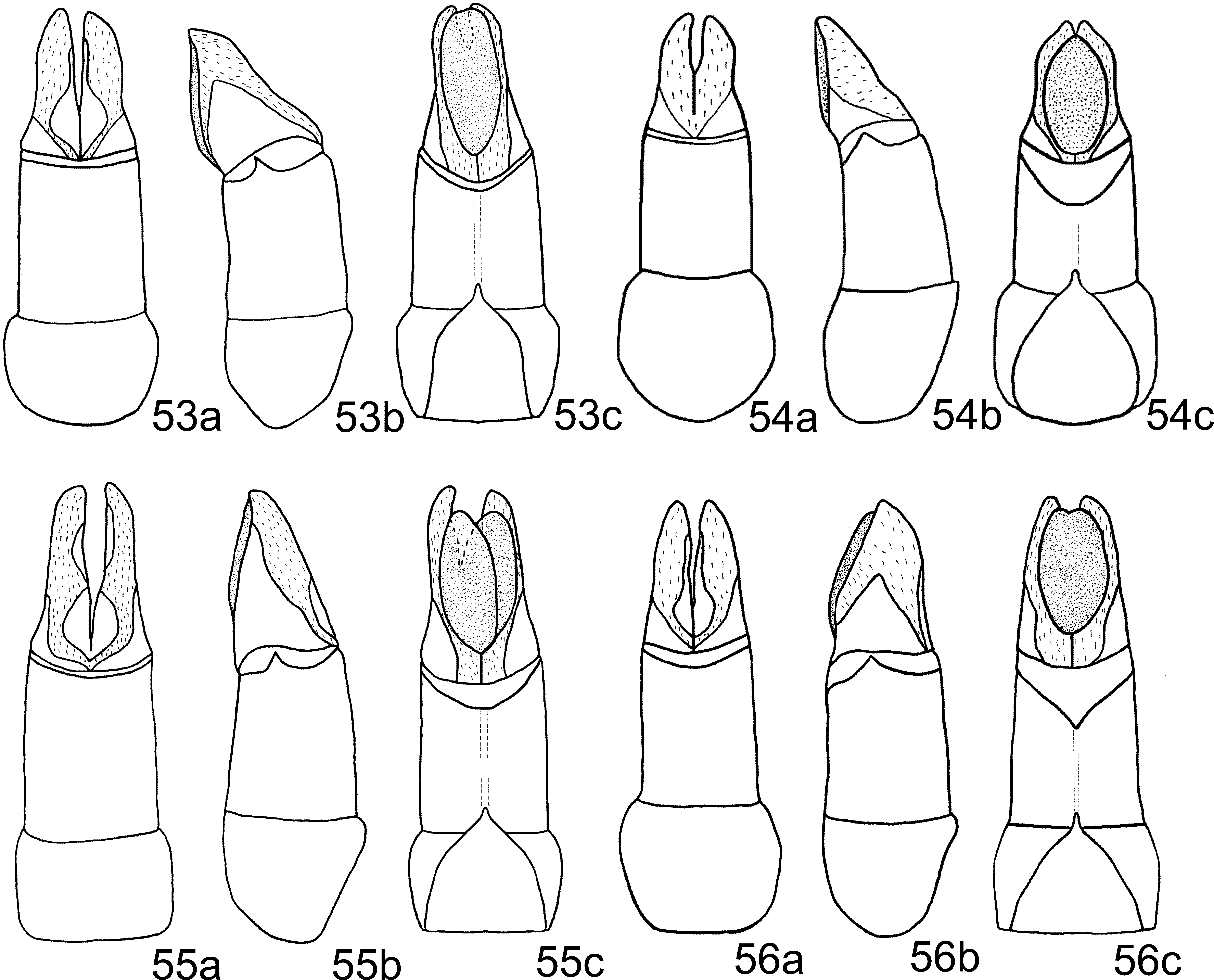
Aedeagus: Phallobase, in ventral view, with apical membranous area only slightly wider ( Fig. 55 View FIGURES 53 – 56 c). Medial sclerite only slightly curved ( Fig. 59 View FIGURES 57 – 62 ).
Measurements: Males (43 specimens): TL: AV: 13.49; MX: 17.4: MN: 9.3; SD: 1.65. PL: AV: 11.78; MX: 14.6; MN: 8.4; SD: 1.41. PW: AV: 8.08; MX: 10.5; MN: 5.6; SD: 1.04. Females (44 specimens): TL: AV: 13.56; MX: 16.6; MN: 9.9; SD: 1.46. PL: AV: 11.79; MX: 13.8; MN: 8.8; SD: 1.21; PW: AV: 8.15; MX: 9.8; MN: 6; SD: 0.88. Total (87 specimens): TL: AV: 13.53; SD: 1.55. PL: AV: 11.77; SD: 1.3. PW: AV: 8.11; SD: 0.96.
Intraspecific variation and taxonomic discussion: Much of the intraspecific variation of G. inermis is a function of size. Very small specimens have the cephalic projection almost entirely reduced, showing only a carina slightly arched upward; in major specimens, on the other hand, this projection rises beyond that, but never as in G. aeruginosa and G. lemoinei ( Fig. 32 View FIGURES 29 – 33 ). In minor specimens the “pronotal hump” is not evident (Fig. 20c), while in the major specimens the anterior region of the pronotum is notably swollen (Fig. 20a, white arrow). The elevation of the sutural margin of elytra is more obvious in larger specimens than in small ones (Fig. 20a, blue arrow).
Most specimens of G. inermis have no posterior pronotal fossae, as in G. amazonica . However, we examined 11 specimens of both sexes that have a pair of weak circular impressions in the posterior region of pronotum, but differ from the posterior fossae of G. aeruginosa , G. lemoinei , and G. dichroa in position and being much smaller ( Fig. 43 View FIGURES 34 – 48. 34 ). These specimens have “partial length” between 8.4 and 11.3 mm and pronotal width between 5.6 and 7.8 mm, i.e., measurements below the average for G. inermis . Interestingly, other specimens in the same size range do not have any trace of these fossae. D’Olsoufieff (1924) was the first to observe the presence of fossae in this species, claiming to have seen them in two small specimens from Mato Grosso, Brazil. Despite not having found any other morphological distinction with the rest of the species, he proposed the “ var. bipunctata ” for these specimens based only on the presence of these fossae (“ Deux petits exemplaires de Matto-Grosso ont deux fossettes basales du pronotum petites mais bien marquées, ce que je n’ai pas observé chez les autres. Le matériel manquant d’un côté et n’ayant pas trouvé d’autres différences avec les lacordairei typiques, je ne les compte pas en espèce distincte (…). Je me borne, pour les exemplaires cités, à leur donner le nom var. bipunctata nov.”). Following the Article 45.6.4 of the Code, as the name “ bipunctata ” was expressly described as a variety by d’Olsoufieff, it must be regarded as of subspecific category and thus available. Nevertheless, as we observed that these fossae are present throughout the distribution of G. inermis (Porto Murtinho, Selvíria, Seropédica, Curitiba, Timbó, Torres, Santiago del Estero, Córdoba and Bañado de Medina), are always restricted to small specimens, and by the absence of any other distinctive characters, we believe that it is only a matter of individual variability and therefore propose synonymy with the nominotypical subspecies. Similarly, two specimens of G. amazonica examined by us also have a pair of these fossae; however, one has above average size for that species, indicating that, at least in G. amazonica , they are not restricted to small specimens (but nonetheless simple individual variability).
Sexual dimorphism in this species is easily observable, contrary to Barattini & Saénz (1964): “ la morphologie externe de cette espèce n’est pas affectée par le sexe, seuls les caractères sexuels primaires permettent de les différencier ”. Among other sexual characteristics of Gromphas , it is possible to determine the sex of G. inermis by examining the ventral carina of the protibia, which is simple in females ( Fig. 23 View FIGURES 21 – 23 a) and with a row of tubercles in males (Figs. 20d, 23b). Interestingly, Barattini & Saenz (1964) mentioned this characteristic, but did not recognize it as a male sexual feature. Only in very worn specimens these tubercles are difficult to see.
Unique features of G. inermis are: (a) posterior margin of pronotum projected at middle ( Figs. 15 View FIGURES 13 – 15. 13 a–c); (b) metatibial spur straight at apex ( Fig. 40 View FIGURES 34 – 48. 34 ) (in all other species it is distinctly curved [ Fig. 39 View FIGURES 34 – 48. 34 ]); (c) and sutural margin of elytra clearly raised in its apical half (Fig. 20a, blue arrow). The pronotal hump is also limited to large specimens of G. inermis (Fig. 20a, white arrow). As in G. aeruginosa and especially G. lemoinei , the pronotum of G. inermis has granulation evident at center (in G. amazonica and G. dichroa , granulation is absent). Gromphas inermis shares only with G. lemoinei a densely punctate sutural margin of elytra and metasternum (in G. amazonica the punctation is also dense, but never at the same density of these two species). See comparison with G. amazonica and G. dichroa in the discussion of these respective species.
Comments: Although we have found only one specimen that was undoubtedly part of the type series of G. inermis , we know that Harold (1869b) based his description in more than one specimen because he gave a size range (“ long. 12–15 mill. ”), mentioned sexual dimorphism and cited more than one collection locality (“ St. Catharina , Buenos Aires, Corrientes ” and “ G. inermis findet sich in Montivideo und Buenos Aires, dann im südlichen Brasilien von Porto Allegro bis Santa Catarina ”). Thus, we designate this specimen as the lectotype of G. inermis ; the paralectotypes are unknown to us.
D’Olsoufieff (1924) made clear that he based his description of G. lacordairei var. bipunctata in two specimens from Mato Grosso, Brazil. However, we were able to locate only one of these specimens at MNHN. Here, we designate this specimen as the lectotype of G. lacordairei bipunctata ; the paralectotype is unknown to us.
Judulien (1899) briefly described the larva of G. inermis . His observation that it bears five antennomeres contradicts Edmonds & Halffter (1978), who observed that all known larvae of Scarabaeinae have four antennomeres.
Bionomics: Brullé (1837) was the first author to publish information on the natural history of Gromphas , probably referring to G. inermis . According to him, Lacordaire noted that this species burrows beneath horse dung. Subsequent authors (Judulien 1899, Fabre 1919, Halffter & Matthews 1966, Halffter & Edmonds 1982, Sánchez & Genise 2008) studied more thoroughly the nesting behavior of this species. Gromphas inermis has been also reported as a consumer of human, equine, bovine, and ovine dung in pastures (Lange 1947, Flechtmann & Rodrigues 1995, Flechtmann et al. 1995, Walsh & Cordo 1997, Morelli et al. 2002, Hamel-Leigue et al. 2006, Silva et al. 2009), and one specimen was collected by Louzada et al. (1996) using rotten cow spleen as bait. Flechtmann et al. (1995) and Monteresino et al. (1996) reported that G. inermis is diurnal. This species was also recognized as an intermediate host of the acanthocephalan worm Macracanthorhynchus hirudinaceus (Pallas, 1781) , a parasite of the domestic pig, Sus scrofa Linnaeus, 1758 (Manter 1928, Lima 1953, Martínez 1959, Monteresino et al., 1996). Our data indicate that G. inermis is collected throughout the year.
A curious pattern of edaphic preference was observed between G. inermis and Ontherus sulcator (Fabricius, 1775) in two studies. Flechtmann & Rodrigues (1995), studying the fauna of dung beetles of Jaraguá do Sul (Santa Catarina, Brazil), observed a clear differential distribution between these two species; while G. inermis was found only in flooded areas, O. sulcator was largely confined to sloping areas with no accumulation of water. Likewise, Sánchez & Genise (2008), working at Navarro (Buenos Aires, Argentina), noted that G. inermis was more abundant around a lake, while O. sulcator was predominant on higher ground. As pointed out by Flechtmann & Rodrigues (1995), it is likely that water saturation of the soil influences the spatial distribution of these species. Edaphic preference has been reported for other Phanaeini, in particular for North American species of Phanaeus (e.g., Fincher 1973, Blume & Aga 1978, Edmonds 1994, Rasmussen 1994).
Geographic distribution: Amazon subregion: Pantanal. Chacoan subregion: Cerrado, Chaco, and Pampa. Parana subregion: Brazilian Atlantic Forest, Parana Forest, and Araucaria angustifolia Forest. BRAZIL: Bahia: Mucuri. Mato Grosso: Chapada dos Guimarães, Nobres, Poconé, Rosário Oeste, Santo Antônio de Leverger. Mato Grosso do Sul: Aquidauana, Campo Grande, Corumbá, Porto Murtinho, Selvíria. Minas Gerais: Pouso Alegre. Espírito Santo: Linhares, São Mateus (Ilha de Guriri). Rio de Janeiro: Armação de Búzios, Cabo Frio, Duque de Caxias, Rio de Janeiro, São Gonçalo, Seropédica. São Paulo: Araçatuba, Cássia dos Coqueiros, Indiana, Piracicaba, Ribeirão Preto, São José do Rio Preto. Paraná: Castro, Curitiba, Foz do Iguaçu, Ivaí, Lapa, Marechal Cândido Rondon, Ponta Grossa. Santa Catarina: Blumenau, Canoinhas, Corupá, Jaraguá do Sul, Joinville, Mafra, Rio Negrinho, Rodeio, São Bento do Sul, Seara, Timbó. Rio Grande do Sul: Aceguá, Bagé, Mostardas, Pelotas, Porto Alegre, Santo Augusto, Torres, Tramandaí. BOLIVIA: Beni. Santa Cruz: Chiquitos. Tarija (Tarija).
PARAGUAY: Amambay: Pedro Juan Caballero. San Pedro. Caaguazú: Caaguazú. Distrito Capital: Assunção. Paraguarí: Ybycuí. Guairá: Colonia Independencia, Villarrica. Caazapá: Caazapá. Itapúa: Coronel Bogado. ARGENTINA: Salta: Guachipas, Salta. Formosa: Guaycolec. Chaco. Santiago del Estero: Río Hondo (Termas do Río Hondo). Misiones. Corrientes: Ituzaingó, Santo Tomé. Santa Fé. Córdoba: Río Primero (La Para), San Justo (Miramar). Entre Ríos. Buenos Aires: Florencio Varela, La Plata, Navarro, Puán, San Isidro (Boulogne Sur Mer). Ciudad Autónoma de Buenos Aires. URUGUAY: Artigas. Rivera: Vichadero; Tacuarembó. Cerro Largo: Melo. Durazno. Flórida. Maldonado. Montevidéu. ( Fig. 66 View FIGURE 66 ).
Material examined: 244 males and 211 females (590 with undetermined sex). ARGENTINA: 1939, Parko col.— 1 female (MNRJ). BUENOS AIRES: La Plata, without date and collector— 3 males and 1 female (MLPA); Puán, F. Sola, I.1959, A. Martínez col.— 2 males and 4 females (MZSP). CIUDAD AUTÓNOMA DE BUENOS AIRES: I.1993, without collector— 1 male (CEMT). CHACO: without date and collector— 1 male (MZSP). CÓRDOBA: 16.III.1939, without collector— 1 male (MNRJ); 04.IV.1939, without collector— 1 male (MNRJ); Río Primero, La Para, X.1986, Z. Monteresino col.— 1 male (CEMT). CORRIENTES: Santo Tomé, XII.1925, without collector— 1 male (MLPA). FORMOSA: Guaycolec, II.1949, A. Martínez col.— 2 males (MZSP). SALTA: Guachipas, I.1951, A. Martínez col.— 1 female (MZSP). SANTIAGO DEL ESTERO: without date, Wagner col.— 4 males and 2 females (MLPA); Rio Salado, without date, Wagner col— 1 male (MLPA). BOLIVIA: TARIJA: Tarija, between Yaguacua-Caiza, 21º50’52’’S 63º36’26’’W, 620m, 03.I.2005, Mann, Hamel & Herzog cols.— 1 male and 1 female (CEMT). BRAZIL: BAHIA: Mucuri, I.1999, G. L. D. Leite col.— 1 male (CEMT). ESPÍRITO SANTO: Linhares, X.1995, T. M. Virgens col.— 1 female (CEMT). MATO GROSSO: Nobres, XI.1985, without collector— 1 female (CEMT); Poconé, Fazenda Alvorada, 16º26’53”S 56º24’44”W, 21.XI.2011, M. B. Pessoa col.— 1 male (CEMT); Rosário Oeste, without date and collector— 2 males and 2 females (MNRJ); Rosário Oeste, without date, Dirings col.— 1 male (MZSP); Santo Antonio do Leverger, Fazenda Vale Esperança, 20.X.1990, Marinez Marques col.— 1 female (CEMT). MATO GROSSO DO SUL: Aquidauana, VII.2011, C. M. A. Correa col.— 2 females (CEMT); Campo Grande, 1990-1992, I. Bianchin col.— 1 male (CEMT); Campo Grande, Jaraguá, XII.1936, W. Zikán col.— 1 male and 1 female (MNRJ); Corumbá, BEP/UFMS, 19º33’53”S 57º00’39”W, 06.X.2011, M. B. Pessoa col.— 6 males and 2 females (CEMT); Corumbá, Passo da Lontra, BEP/UFMS, without date, V. Lopes col.— 1 male (CEMT); Porto Murtinho, XII.1929, without collector— 1 male (MZSP); Porto Murtinho, I.1930, without collector— 1 male and 1 female (MZSP); Riacho do Herval, Rio Paraná, XII.1951, B. Pohl col.— 4 males (MZSP); Selvíria, Fazenda UNESP, 21.XI.1992, C. A. H. Flechtmann col.— 1 female (CEMT); Três Marias, margem esquerda do rio Sucuriú, Fazenda Caimã, X.1966, F. Lane col.— 1 female (MZSP). MINAS GERAIS: Pouso Alegre, 27.XII.1958, M. Vogas col.— 1 male (MNRJ). PARANÁ: Curitiba, III.1898, without collector— 1 female (MZSP); Curitiba, 25.XII.1936, C. Westerman col.— 3 males (MZSP); Curitiba, XI.1941, B. Pohl col.— 2 males (MZSP); Curitiba, II.1944, B. Pohl col.— 1 male (MZSP); Ivaí, Rio Ivaí, II (without year), W. Kosak col.— 1 female (MNRJ); Marechal Cândido Rondon, Porto Artaza, without date and collector— 1 female (MZSP); Parque Nacional do Iguaçu, XI.1992, A. F. A. Luna Dias col.— 1 female (FIOC). RIO DE JANEIRO: Armação de Búzios, Praia Rasa, XII.1995, L. H. Gil Azevedo col.—1 sem sexo (DZRJ); Cabo Frio, VII.1980, Jane M. Costa col.— 1 male (FIOC); Duque de Caxias, IX.1990, F. Z. Vaz-de-Mello col.— 1 female (CEMT); Duque de Caxias, São Bento, VIII.1960, P. A. Teles col.— 1 female (MNRJ); Rio de Janeiro, Copacabana, XI.1990, F. Z. Vazde-Mello col.— 1 female (CEMT); Rio de Janeiro, Copacabana, XII.1992, R. L. Vaz-de-Mello col.— 1 female (FIOC) and 1 female (CEMT); Rio de Janeiro, Copacabana, XII, 1993, F. Z. Vaz-de-Mello col.— 1 male and 1 female (CEMT); Rio de Janeiro, Copacabana, XII.1994, R. L. Vaz-de-Mello col.— 1 female (FIOC) and 2 males (CEMT); Rio de Janeiro, Jacarepaguá, IX.1990, F. Z. Vaz-de-Mello col.— 1 female (FIOC); Rio de Janeiro, Marapendi, XI.1987, Hugo col.— 1 female (CEMT); São Gonçalo, X.1982, Pêssoa col.—1 sem sexo (DZRJ); Seropédica, Estrada Rio-São Paulo, km 47, 30.VII.1951, J. F. Zikán Neto col.— 1 male (MNRJ); Seropédica, Universidade Federal Rural do Rio de Janeiro (UFRRJ), 21.X.1990, A. Saraiva col.— 1 male and 1 female (FIOC); Seropédica, UFRRJ, 17.XI.1990, without collector— 2 males (FIOC). RIO GRANDE DO SUL: III.1915, without collector— 1 female (MZSP); Mostardas, I.1945, Pe. Buck col.— 1 male (MZSP); Aceguá, Fazenda Centinela, 31º27’30”S 54º21’18”W, 08-14.XI.2011, R. M. Moraes col.— 2 males and 5 females (CEMT); Bagé, Embrapa/ CPPSUL, 06.XII.2006, L. D. Audino col.— 1 female (CEMT); Bagé, Embrapa/CPPSUL, 17.I.2007, L. D. Audino col.— 1 male (CEMT); Bagé, Fazenda Santo Antônio, 07-13.I.2012, R. M. Moraes col.— 2 males and 2 females (CEMT); Pelotas, I.1935, without collector— 1 male and 1 female (MNRJ); Pelotas, XII.1934, without collector— 1 male and 1 female (MNRJ); Porto Alegre, without date and collector— 1 male (MNRJ); Porto Alegre, VI.1927, without collector— 1 female (MZSP); Torres, 09.XII.1964, Pe. P. Buck col.— 1 male (CEMT); Tramandaí, I.1979, C. Coimbra Jr. col.— 1 male (MZSP). SANTA CATARINA: Blumenau, XI.1924, without collector— 1 male (MZSP); Canoinhas, Pinhal, XI.1951, A. Maller col.— 1 female (MNRJ); Canoinhas, Pinhal, XII.1952, A. Maller col.— 2 females (MNRJ); Corupá, V (without year), Anton Maller col.— 1 male (MNRJ); Corupá, X (without year), Anton Maller col.— 1 male (MNRJ); Corupá, XII.1953, A. Maller col.— 1 male (MNRJ); Joinville, VI.1899, without collector— 1 female (MZSP); Mafra, without date and collector— 1 male (MNRJ); Rio Negrinho, XI.1925, A. Maller col.— 1 male and 1 female (MNRJ); Rio Vermelho, without date, Dirings col.— 1 male and 1 female (MZSP); Rio Vermelho, IV.1963, Dirings col.— 3 males and 1 female (MZSP); Rio Vermelho, III.1964, Dirings col.— 1 female (MZSP); Rodeio, Rio Benedito, without date, Dirings col.— 6 males and 2 females (MZSP); Seara, Nova Teutônia, I.1966, F. Plaumann col.— 1 male (MZSP); São Bento do Sul, I.1948, Dirings col.— 1 male (MZSP); Timbó, II.1952, Dirings col.—42 with undetermined sex (MZSP); Timbó, V.1956, Dirings col.— 22 males and 24 females (MZSP); Timbó, II.1958, Dirings col.—104 with undetermined sex (MZSP); Timbó, XI.1965, Dirings col.—161 with undetermined sex (MZSP); Timbó, XII.1956, Dirings col.— 9 males and 11 females (MZSP); Timbó, V.1962, Dirings col.— 37 males, 34 females and 190 with undetermined sex (MZSP); Timbó, III.1965, Dirings col.— 78 males, 70 females and 92 outros with undetermined sex (MZSP). SÃO PAULO: Araçatuba, Anhangaí, XII.1926, without collector— 1 male (MZSP); Cássia dos Coqueiros, II.1955, M. P. Barreto col.— 2 males (MZSP); Indiana, 10.II.1935, without collector— 1 female (MNRJ); Piracicaba, without date and collector— 1 male and 1 female (MZSP); Ribeirão Preto, XII.1954, Barreto col.— 2 females (MZSP); Ribeirão Preto, VIII.1955, E. X. Rabello col.—1 espécime (MZSP); São José do Rio Preto, I.1932, B. Pohl col.— 1 female (MZSP). PARAGUAY: AMAMBAY: Pedro Juan Caballero, XI.1998, Maruíz Díaz col.— 2 males (CEMT). GUAIRÁ: Colonia Independencia, I.1950, Foerster col.— 1 female (MNRJ); Colonia Independencia, III.1950, Juan Foerster col.— 1 male (MNRJ). ITAPÚA: Coronel Bogado, I.1944, Martínez col.— 1 female (FIOC). URUGUAY: without date and collector— 1 male (MZSP). ARTIGAS: Arroy Tres Cruces Grande, 18.I-20.II.1958, M. A. Monné col.— 1 female (MZSP). CERRO LARGO: Bañado de Medina, 13-20.XII.2011, R. M. Moraes col.— 4 males and 4 females (CEMT); Melo, Fazenda La Invernada, 13-20.XII.2011, R. M. Moraes col.— 1 male and 1 female (CEMT). MONTEVIDEO: Montevideo, without date and collector— 1 male (MZSP). RIVERA: Vichadero, Fazenda El Cerro, 21-27.XI.2011, R. M. Moraes col.— 1 male and 1 female (CEMT).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Scarabaeinae |
|
Tribe |
Phanaeini |
|
Genus |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Scarabaeinae |
|
Tribe |
Phanaeini |
|
Genus |